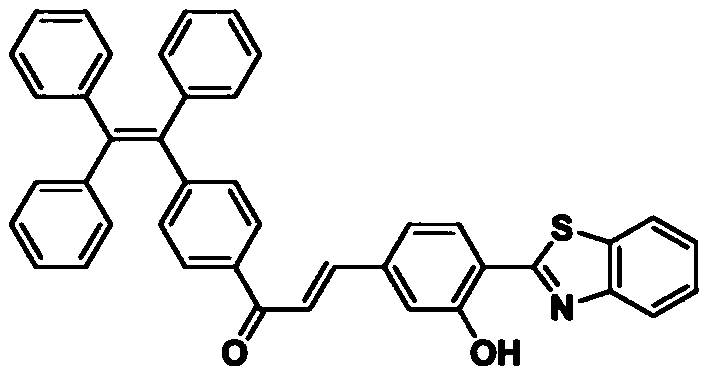
Fungus fluorescent staining solution and preparation method thereof
A fluorescent dyeing and fungal technology, which is applied in the field of biological testing of pathogenic bacteria in medical clinical testing, can solve the problems that the components of the dyeing solution cannot coexist stably for a long time, the stability of the fluorescent whitening agent is poor, and the stability of the fluorescein is affected, so as to achieve good clinical application. Foreground, improve staining effect and staining durability, avoid the effect of cell self-absorption and self-fluorescence interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A fungal fluorescent staining liquid, comprising the following components by weight: 0.8 parts of fluorescent whitening agent 28, 0.05 parts of eosin, 0.02 parts of hematoxylin, 10 parts of dimethyl sulfoxide, 0.5 parts of glycerin, and 0.3 parts of propyl gallate , 0.2 part of ascorbic acid, 0.3 part of surfactant, 0.8 part of sodium chloride.
[0037] The preparation method of above-mentioned fungal fluorescent staining liquid, comprises the following steps:
[0038] (1) Weigh dimethyl sulfoxide, glycerin, propyl gallate, ascorbic acid, surfactant and sodium chloride in corresponding weight proportions, add them into deionized water successively, stir to obtain solution A, and set aside;
[0039] (2) Weigh the fluorescent whitening agent 28 of the corresponding weight and add it into deionized water to fully stir and dissolve to obtain solution B for subsequent use;
[0040] (3) Weigh the corresponding weight of eosin and hematoxylin and add it into deionized water t...
Embodiment 2
[0044] A fungal fluorescent staining liquid, comprising the following components by weight: 1.2 parts of fluorescent whitening agent 28, 0.08 part of eosin, 0.05 part of hematoxylin, 15 parts of dimethyl sulfoxide, 1 part of glycerin, and 0.6 part of propyl gallate , 0.5 parts of ascorbic acid, 0.5 parts of surfactant, and 1.0 parts of sodium chloride.
[0045] The preparation method of above-mentioned fungal fluorescent staining liquid, comprises the following steps:
[0046] (1) Weigh dimethyl sulfoxide, glycerin, propyl gallate, ascorbic acid, surfactant and sodium chloride in corresponding weight proportions, add them into deionized water successively, stir to obtain solution A, and set aside;
[0047] (2) Weigh the fluorescent whitening agent 28 of the corresponding weight and add it into deionized water to fully stir and dissolve to obtain solution B for subsequent use;
[0048] (3) Weigh the corresponding weight of eosin and hematoxylin and add it into deionized water ...
Embodiment 3
[0053] A fungal fluorescent staining liquid, comprising the following components by weight: 1.0 parts of fluorescent whitening agent 28, 0.07 parts of eosin, 0.03 parts of hematoxylin, 13 parts of dimethyl sulfoxide, 0.8 parts of glycerin, and 0.5 parts of propyl gallate , 0.4 part of ascorbic acid, 0.4 part of surfactant, 0.9 part of sodium chloride.
[0054] The preparation method of above-mentioned fungal fluorescent staining liquid, comprises the following steps:
[0055] (1) Weigh dimethyl sulfoxide, glycerin, propyl gallate, ascorbic acid, surfactant and sodium chloride in corresponding weight proportions, add them into deionized water successively, stir to obtain solution A, and set aside;
[0056] (2) Weigh the fluorescent whitening agent 28 of the corresponding weight and add it into deionized water to fully stir and dissolve to obtain solution B for subsequent use;
[0057] (3) Weigh the corresponding weight of eosin and hematoxylin and add it into deionized water t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com