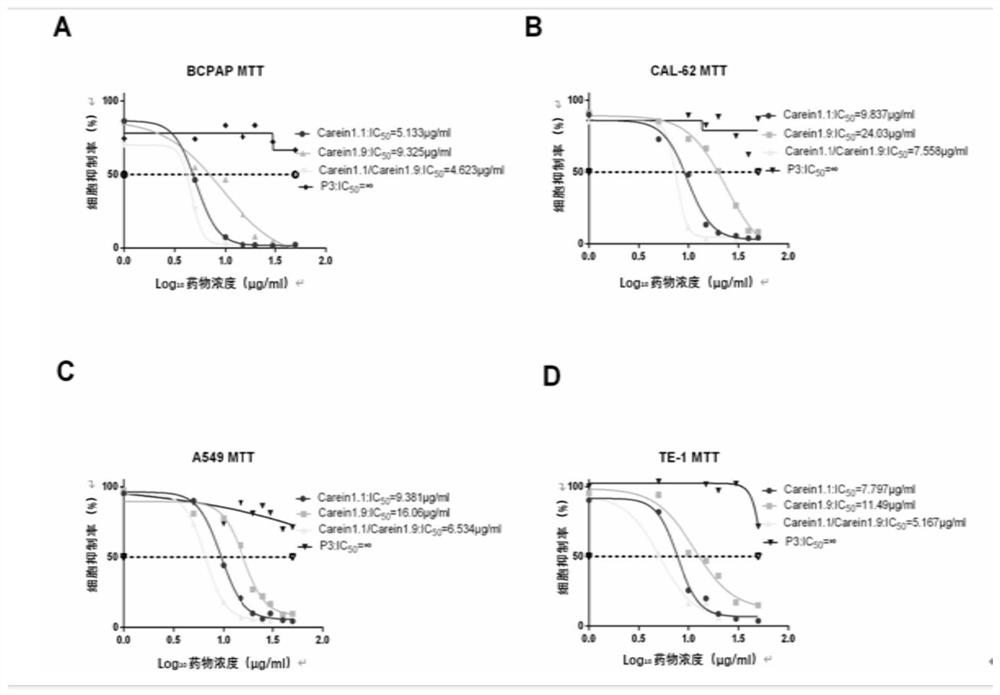
a containing 131 i-labeled caerin1.1 polypeptide and its application
A technology of labeling and polypeptide solution, applied in the biological field, can solve the problems of progression-free survival, patient difficulty, etc., and achieve the effects of low cost, high labeling rate, and simple labeling process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0033] Experimental example 1 cell line and cell culture
[0034] B-CPAP human thyroid cancer cell B-CPAP (papillary) and CAL-62 human thyroid cancer cell CAL-62 (undifferentiated), these two types of cells are provided by the Chinese Academy of Sciences Stem Cell Bank, and the two types of cells are cultured according to the method in the product manual Tie. Wherein, B-CPAP cell culture fluid formula: 87% RPMI Medium 1640 (GIBCO), 10% heat-inactivated fetal bovine serum from New Zealand, 0.1% Penicillin-Streptomycin, Liquid (GIBCO), 1% MEM Non-Essential Amino Acids (NEAA, GIBCO), 1% Glutamax (GIBCO), 1% Sodium Pyruvate (GIBCO); CAL-62 cell culture medium formula: 90% DMEM Mediun (GIBCO), 10% from New Zealand % heat-inactivated fetal bovine serum, 0.1% Penicillin-Streptomycin, Liquid (GIBCO); both cells were stored at 37°C, 5% CO 2 cultured in an incubator.
experiment example 2
[0035] Experimental Example 2 Caerin1.1 Polypeptide Cell Proliferation Experiment
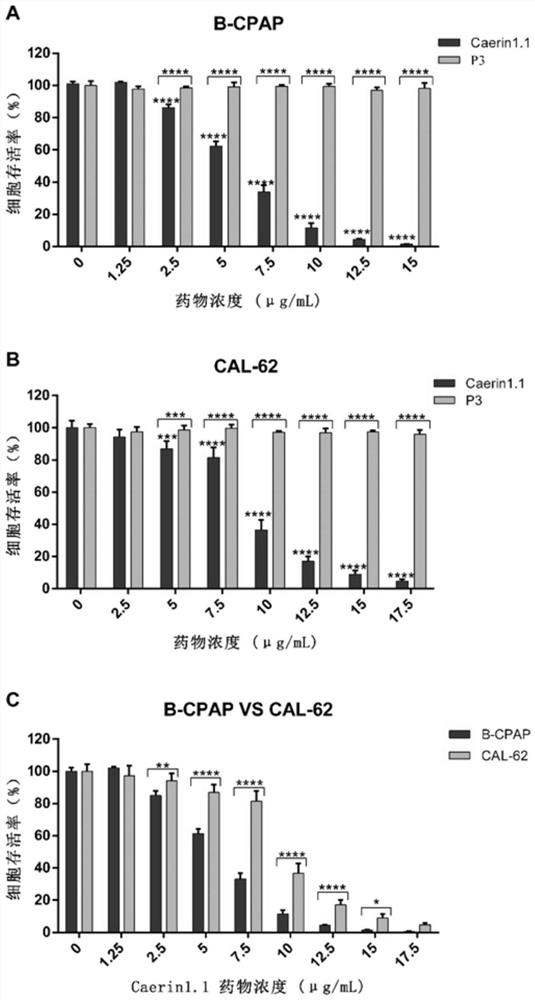
[0036] The activity of B-CPAP cells and CAL-62 cells was induced by 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfonic acid Benzene)-2H-tetrazolium monosodium salt (CCK-8, DOJINDO) detection, operate according to the instructions: B-CPAP cells in the logarithmic growth phase, CAL-62 cells in 5 × 10 per well 3 / 100μL cells were seeded in 96-well cell culture plates, placed at 37°C, 5% CO 2 Under culture for 24 hours, until the cell aggregation rate is about 60-70%. The Caerin1.1 polypeptide and control peptide P3 were added to each well in groups according to different concentrations so that the final concentration was in the range of 0-17.5 μg / mL (0, 1.25, 2.5, 5, 7.5, 10, 12.5, 15, 17.5 μg / mL), at the same time set the zero hole, each group of 4 auxiliary holes, and at 37 ℃, 5% CO 2 Conditioned for 24h. Add 10 μL CCK-8 to each well and incubate for 4-6 hours to stop the exper...
experiment example 3
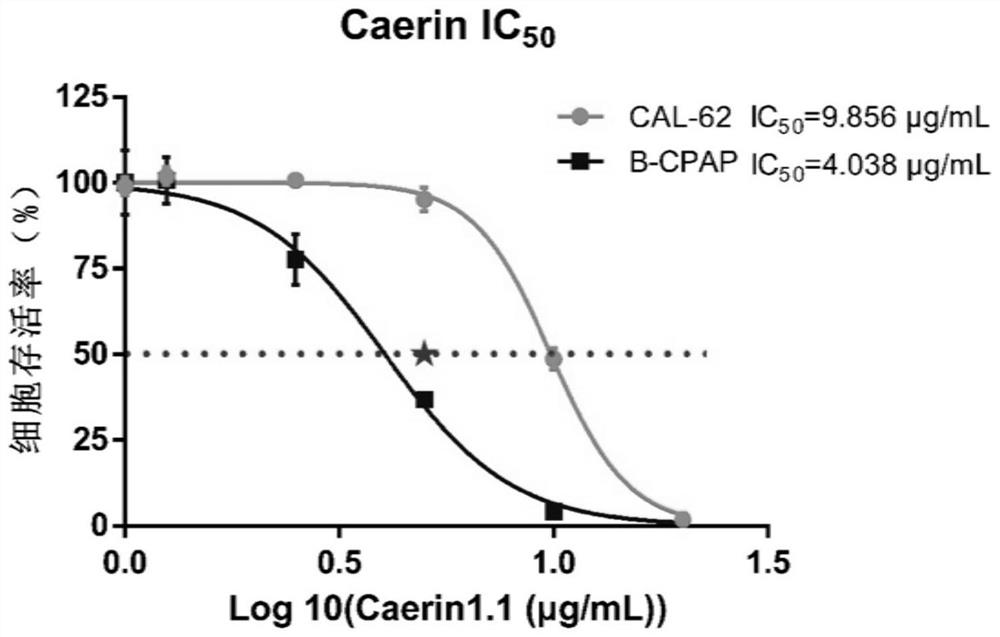
[0038] Experimental Example 3 Caerin1.1 Peptide IC 50 determination
[0039] Logarithmically grown B-CPAP cells and CAL-62 cells were divided into 5×10 3 / 100μL cells were seeded in 96-well cell culture plates at 37°C, 5% CO 2 The cells were cultured for 24 h under the conditions of the above conditions, and they were allowed to adhere to the wall. Add the Caerin1.1 polypeptide to each well according to the concentration gradient of the multiple relationship (0, 1.25, 2.5, 5, 10, 20 μg / mL), and set the zero adjustment well at the same time. %CO 2 Conditioned for 24h. Add 10 μL CCK-8 to each well and incubate for 4-6 hours to stop the experiment. Cell viability was determined by measuring the absorbance (OD) at 450 nm using an enzyme-linked immunosorbent assay. Calculate IC with GraphPad software 50 (half inhibitory concentration).
[0040] see results image 3 , it can be seen that the IC of B-CPAP 50 = 4.038 μg / mL, IC of CAL-62 50 =9.856 μg / mL. Caerin1.1 polypepti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com