A kind of fluorescent dye for lysosome labeling and its synthesis method and application
A technology of a fluorescent dye and a synthesis method, which is applied in the field of lysosome-labeled fluorescent dye and its synthesis, can solve the problems of high phototoxicity, poor structural stability and photostability, limited application, etc., and achieves a simple synthesis method and high efficiency. Fluorescence stability, cheap and easily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthesis of Lyso-DAze, a lysosomal dye.
[0029] Synthesis of intermediate Lyso-NBr:
[0030]
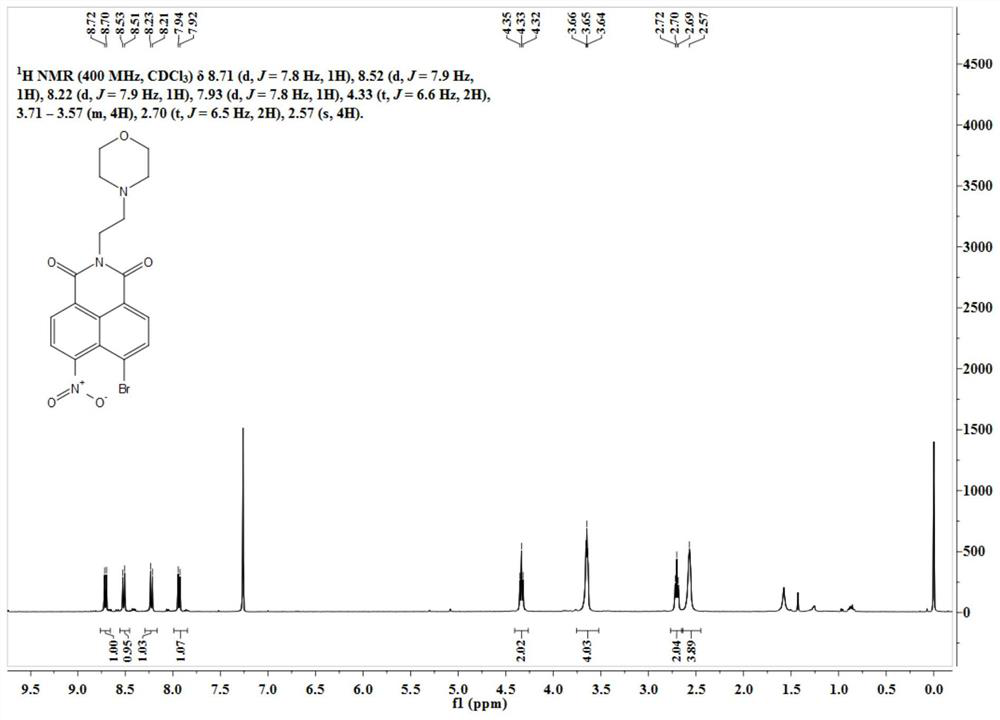
[0031] 4-Bromo-5-nitro-1,8-naphthalimide (0.50g, 1.56mmol) was dissolved in 40mL of ethanol, and N-(2-aminoethyl)morpholine (0.609g, 4.68 mmol). After reacting at 70°C for 3 hours, the solvent was distilled off under reduced pressure, and the residue was separated through a silica gel column (dichloromethane:methanol=200-50:1, V / V) to obtain 1.12 g of a khaki solid with a yield of 55%. Its H NMR spectrum is as figure 1 As shown, the specific data are as follows:
[0032] 1 H NMR (400MHz, CDCl 3 )δ8.71(d, J=7.8Hz, 1H), 8.52(d, J=7.9Hz, 1H), 8.22(d, J=7.9Hz, 1H), 7.93(d, J=7.8Hz, 1H) ,4.33(t,J=6.6Hz,2H),3.71–3.57(m,4H),2.70(t,J=6.5Hz,2H),2.57(s,4H).
[0033] Synthesis of the dye Lyso-DAze:
[0034]
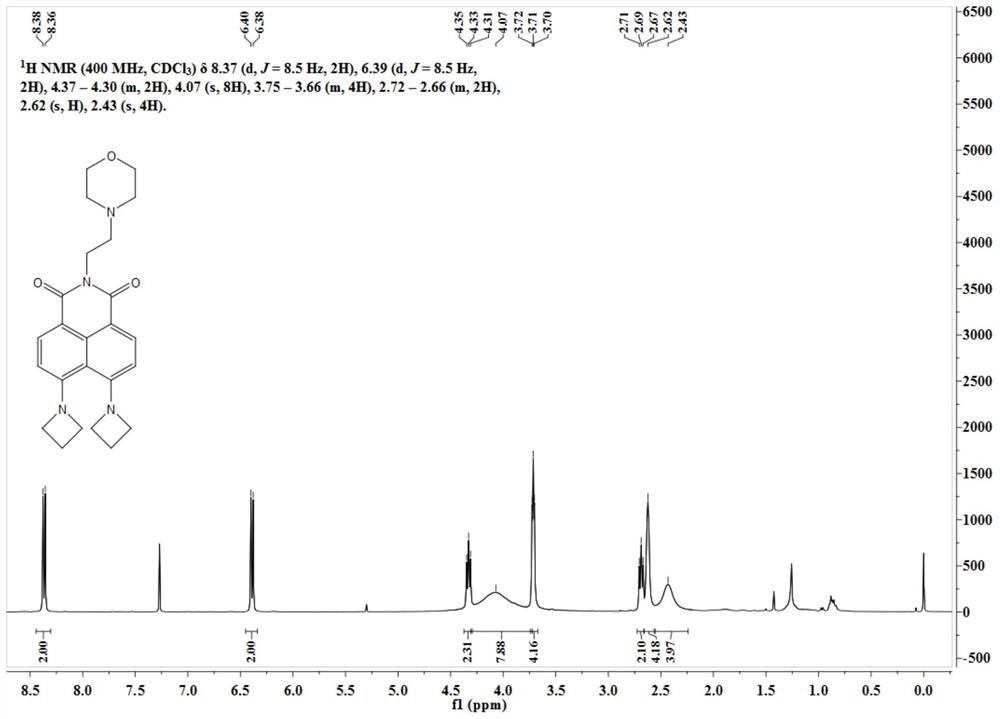
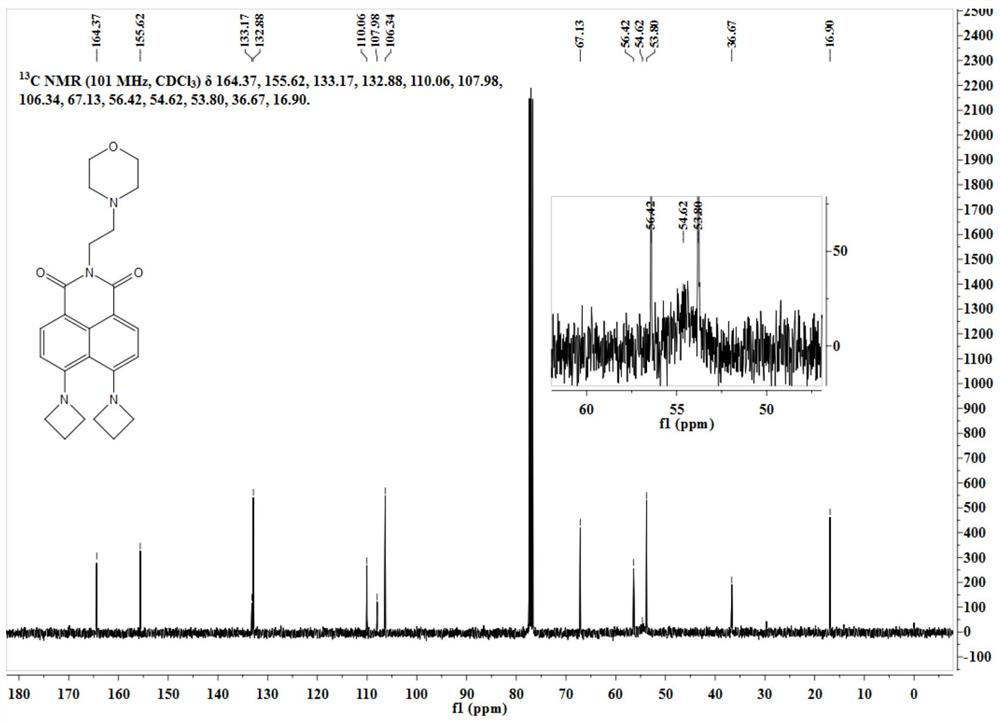
[0035] The intermediate Lyso-NBr (50 mg, 0.12 mmol) was dissolved in 10 mL of ethylene glycol methyl ether, and 100 mg of azetidine was added thereto. The reaction ...
Embodiment 2
[0044] Synthesis of Lyso-DAze, a lysosomal dye.
[0045] Synthesis of intermediate Lyso-NBr:
[0046]
[0047]4-Bromo-5-nitro-1,8-naphthalimide (0.75g, 2.34mmol) was dissolved in 30mL ethanol, and N-(2-aminoethyl)morpholine (0.45g, 3.46 mmol). After reacting at 140°C for 10 h, the solvent was distilled off under reduced pressure, and the residue was separated through a silica gel column (dichloromethane:methanol=200-50:1, V / V) to obtain 0.47 g of a khaki solid with a yield of 46%.
[0048] Synthesis of the dye Lyso-DAze:
[0049]
[0050] The intermediate Lyso-NBr (75 mg, 0.18 mmol) was dissolved in 75 mL of ethylene glycol methyl ether, and azetidine (75 mg, 1.31 mmol) was added thereto. The reaction solution was slowly heated to 160°C and reacted for 30h. Ethylene glycol methyl ether was removed under reduced pressure, and the residue was separated through a silica gel column (dichloromethane:methanol=50:1, V / V) to obtain 7 mg of a yellow solid, with a yield of 10%...
Embodiment 3
[0054] Synthesis of Lyso-DAze, a lysosomal dye.
[0055] Synthesis of intermediate Lyso-NBr:
[0056]
[0057] 4-Bromo-5-nitro-1,8-naphthalimide (0.9g, 2.81mmol) was dissolved in 108mL ethanol, and N-(2-aminoethyl)morpholine (2.16g, 16.60 mmol). After reacting at 120°C for 8 hours, the solvent was distilled off under reduced pressure, and the residue was separated through a silica gel column (dichloromethane:methanol=200-50:1, V / V) to obtain 0.51 g of a khaki solid with a yield of 42%.
[0058] Synthesis of the dye Lyso-DAze:
[0059]
[0060] The intermediate Lyso-NBr (100 mg, 0.24 mmol) was dissolved in 40 mL of ethylene glycol methyl ether, and azetidine (400 mg, 7 mmol) was added thereto. The reaction solution was slowly heated to 140°C and reacted for 24h. Ethylene glycol methyl ether was removed under reduced pressure, and the residue was separated through a silica gel column (dichloromethane:methanol=50:1, V / V) to obtain 8 mg of a yellow solid with a yield of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com