Anti-inflammatory drug for surgical postoperative nursing and preparation method thereof
A technology of anti-inflammatory drugs and compositions, applied in the direction of anti-inflammatory agents, drug combinations, pharmaceutical formulations, etc., can solve problems such as changes in blood images, organ dysfunction, and long-term prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] An anti-inflammatory pharmaceutical composition for postoperative care, comprising the following raw material components in percentage by weight: 4% celecoxib, 1% tetrandrine, 15% sucrose, 10% sodium carboxymethyl starch, Talc 16%, Corn Starch 54%.
[0029] The anti-inflammatory pharmaceutical composition is a capsule, and its preparation method comprises the following steps:
[0030] 1) take each raw material component by weight percentage, for subsequent use;
[0031] 2) Mix celecoxib, tetrandrine, sodium carboxymethyl starch and corn starch evenly, pass through a 100-mesh sieve, add sucrose aqueous solution to make a soft material, and set aside;
[0032] 3) Pass the soft material through a 30-mesh sieve, granulate, and dry with blast at 50°C;
[0033] 4) Pass through a 20-mesh sieve for granulation, add talcum powder, and mix evenly;
[0034] 5) Fill the obtained granules into the capsule shell and pack.
Embodiment 2
[0036] An anti-inflammatory pharmaceutical composition for surgical postoperative care, comprising the following raw material components in percentage by weight: celecoxib 3%, tetrandrine 2%, polyvinylpyrrolidone 20%, glycyl starch sodium 6 %, micronized silica gel 8%, microcrystalline cellulose 61%.
[0037] The anti-inflammatory pharmaceutical composition is a capsule, and its preparation method comprises the following steps:
[0038] 1) take each raw material component by weight percentage, for subsequent use;
[0039] 2) Mix celecoxib, tetrandrine, sodium glycyl starch and microcrystalline cellulose evenly, pass through a 50-mesh sieve, add an aqueous solution of polyvinylpyrrolidone to make a soft material, and set aside;
[0040] 3) Pass the soft material through a 10-mesh sieve, granulate, and dry with blast at 55°C;
[0041] 4) Pass through a 40-mesh sieve for granulation, add micropowder silica gel, and mix evenly;
[0042] 5) Fill the obtained granules into the ca...
Embodiment 3
[0044] An anti-inflammatory pharmaceutical composition for post-surgical care, comprising the following raw material components in percentage by weight: celecoxib 3%, tetrandrine 2%, starch slurry 15%, cross-linked cellulose 8%, Magnesium Stearate 12%, Mannitol 60%.
[0045] The anti-inflammatory pharmaceutical composition is a capsule, and its preparation method comprises the following steps:
[0046] 1) take each raw material component by weight percentage, for subsequent use;
[0047] 2) Mix celecoxib, tetrandrine, cross-linked cellulose and mannitol evenly, pass through an 80-mesh sieve, add an aqueous solution of starch slurry to make a soft material, and set aside;
[0048] 3) Pass the soft material through a 20-mesh sieve, granulate, and blow dry at 52°C;
[0049] 4) Pass through a 30-mesh sieve for granulation, add magnesium stearate, and mix well;
[0050] 5) Fill the obtained granules into the capsule shell and pack.
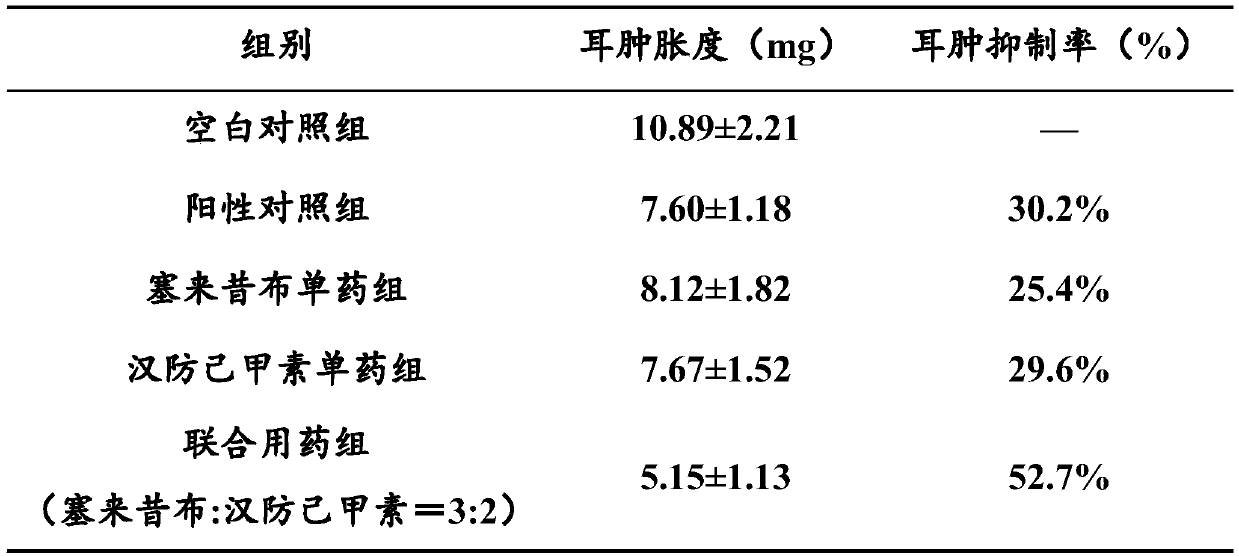
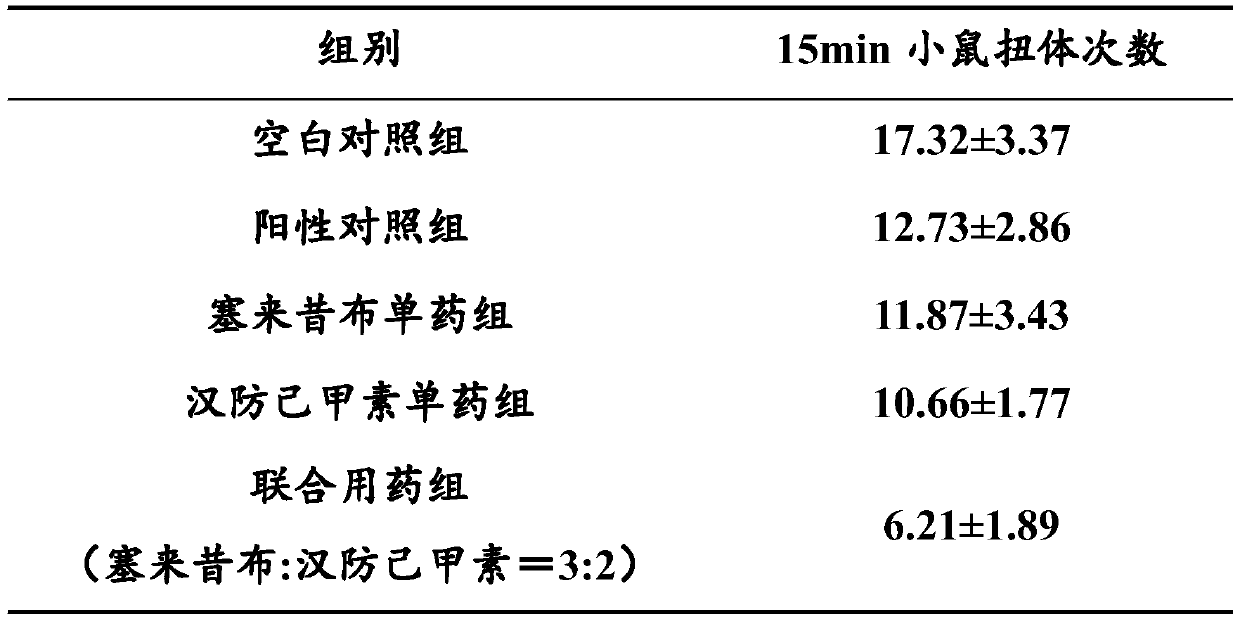
[0051] Anti-inflammatory and analgesic effec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com