Method for recovering and preparing potassium bifluoride by using waste electrolyte generated in fluorine gas production process
A waste electrolyte and production process technology, applied in chemical instruments and methods, electrolysis processes, electrolysis components, etc., can solve the problems of increasing difficulty and separation costs, affecting the recycling of electrolytes, and unreachable content, and achieves low manufacturing costs. The effect of reducing production costs and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
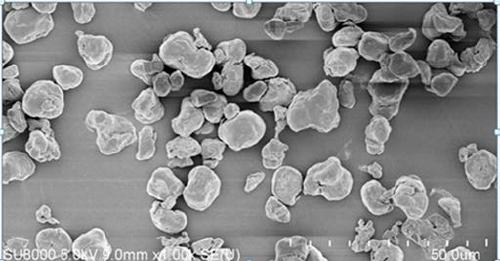
[0031] Take 1000 g of spent electrolyte, heat it to 90°C until it melts completely, and then filter it through a 60-mesh screen, transfer the filtrate into a tetrafluoro bottle, add 1 g of a composition of sodium fluoride and lithium fluoride (wherein sodium fluoride is 0.9 g , lithium fluoride 0.1 g), stirred at a constant temperature of 90°C for 5 hours, then kept at 90°C for 20 hours, filtered with a guanidine-containing PVDF composite filter membrane, maintained the filtrate temperature at 80°C, added 900g of high-purity water while stirring, and stirred until completely After miscibility, add 250g KOH, stir for 2 hours, and filter with guanidine-containing PVDF composite membrane. After filtration, the filtrate is maintained at 80°C, and then begins to cool down and crystallize. Control the cooling rate at 2°C / h, slowly cool down to 5°C, and cool down During the process, the stirring speed was maintained at 40 rpm. After the suspension was centrifuged and dehydrated, the c...
Embodiment 2
[0036] Take 1000 g of spent electrolyte, heat it to 90°C until it melts completely, and then filter it through a 100-mesh screen, transfer the filtrate into a tetrafluoro bottle, add 10 g of a composition of sodium fluoride and lithium fluoride (8.5 g of sodium fluoride, Lithium fluoride 1.5 g), stirred at a constant temperature of 90°C for 5 hours, then kept at 90°C for 24 hours, filtered with a 10-micron PVDF PVDF compound filter membrane containing guanidine, and maintained the filtrate temperature at 85°C, while stirring, add 1000g of high-purity water, stir After complete miscibility, add 285g KOH, stir for 1 hour, and filter with 10 micron PVDF PVDF composite filter membrane containing guanidine. After filtration, the filtrate is maintained at 85°C, and then begins to cool down and crystallize. Control the cooling rate to 2.5°C / h, and slowly cool down To 5°C, maintain the stirring speed at 50 rpm during the cooling process, transfer the crude product to the PTFE tray afte...
Embodiment 3
[0041] Take 1000 g of spent electrolyte, heat it to 95°C until it melts completely, then filter it through a 120-mesh screen, transfer the filtrate into a tetrafluoro bottle, add 20 g of a composition of sodium fluoride and lithium fluoride (18 g of sodium fluoride, Lithium fluoride 2 g), stirred at a constant temperature of 90°C for 5 hours, then kept at 90°C for 24 hours, filtered with a 10-micron PVDF PVDF composite filter membrane containing guanidine, and maintained the filtrate temperature at 80°C, while stirring, add 1050g of high-purity water, stir After it is completely miscible, add 290 g KOH, stir for 1 hour, and filter with a 10-micron PVDF-containing guanidine-containing PVDF composite membrane. After filtration, the filtrate is maintained at 80°C, and then begins to cool down and crystallize. Control the cooling rate at 3°C / h, slowly Cool down to 5°C, keep the stirring speed at 60 rpm during the cooling process, and then transfer the suspension to a PTFE tray afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com