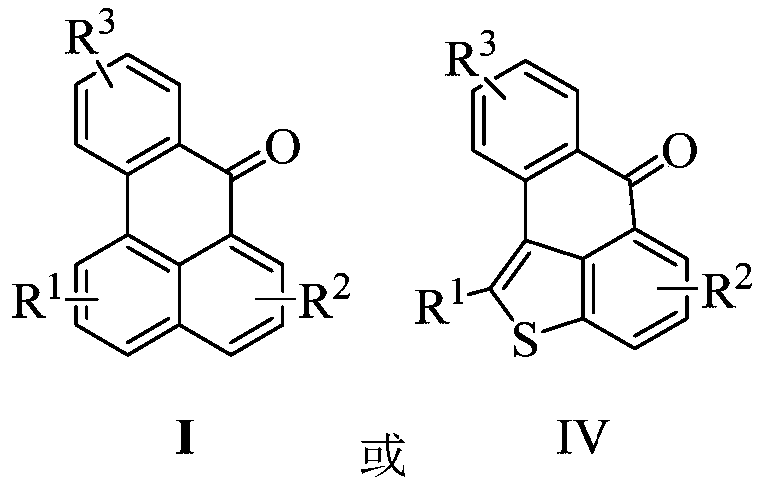
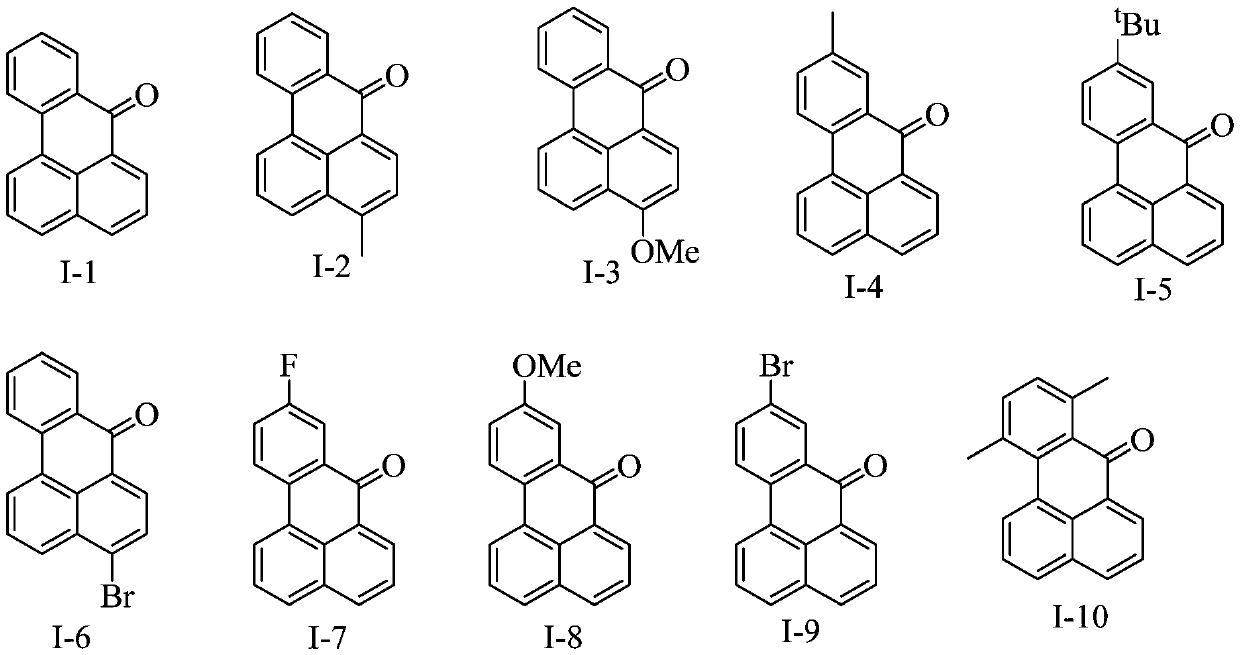
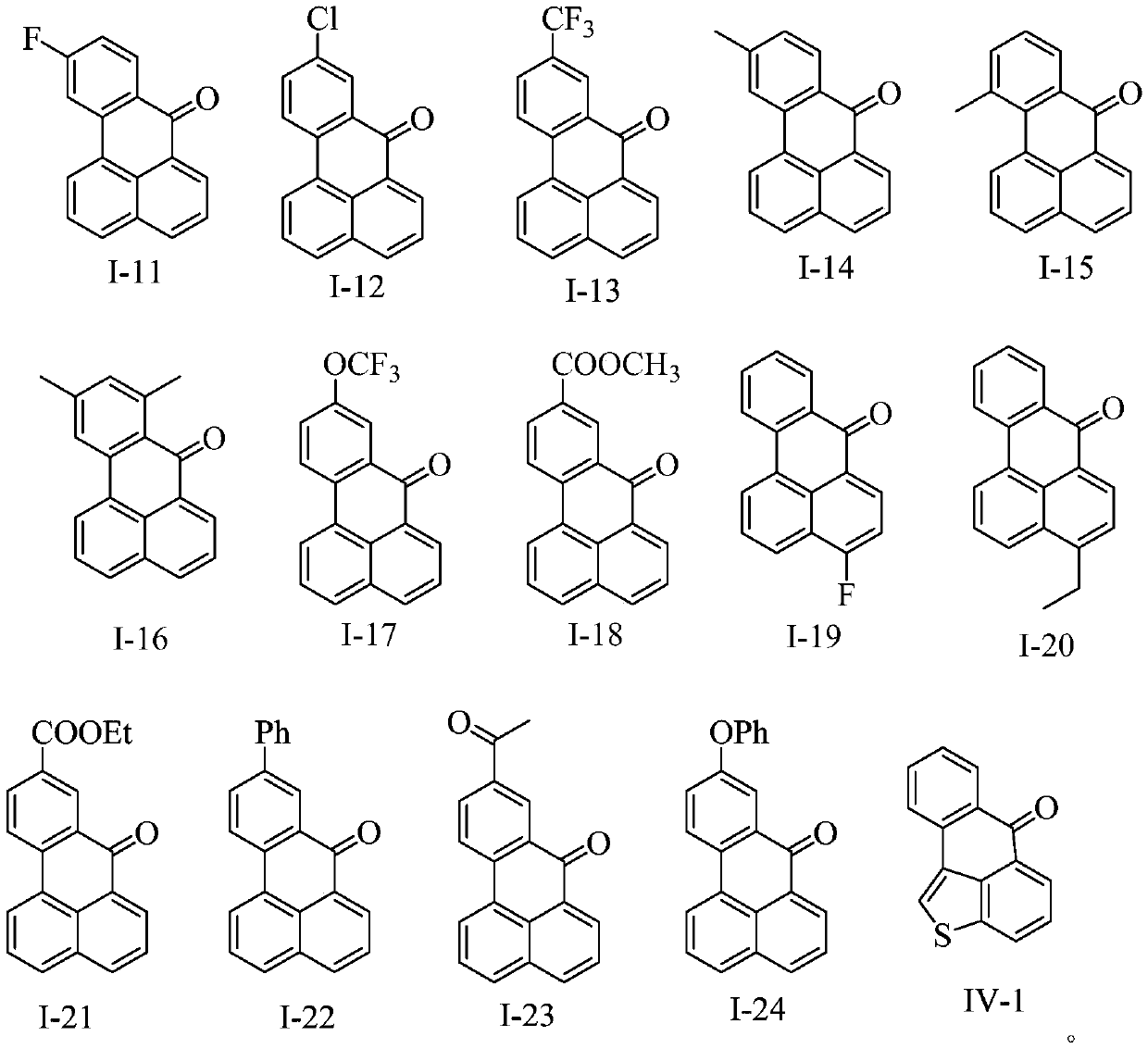
Benzanthrone derivative and preparation method thereof and application thereof in functional pigments
A technology for benzanthrone and derivatives is applied in the field of benzanthrone derivatives and their preparation, and achieves the effects of high optical stability, high thermal stability and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] Preparation of diaryliodonium salts:
[0038]
[0039] Dissolve 1 equivalent of iodobenzene acetate in 10 mL of dichloromethane, drop TfOH (2 equivalents) into the reaction solution at 0°C, and stir the reaction solution at room temperature for 1 h. After 1h, the reaction solution was lowered to 0°C, benzene (1 equivalent) was dropped into the system, and the reaction was continued at room temperature for 2h. After the reaction was completed, the DCM was spin-dried, and anhydrous ether was added, and a white solid was precipitated. The filter cake was washed with water and ether, and dried in vacuum to obtain compound III-1.
[0040]
[0041] Dissolve mCPBA (85%, 22 mmol, 1.1 equiv) and 4-methyliodobenzene (20 mmol, 1.0 equiv) in 10 mL of dichloromethane, and slowly add toluene (122 mmol, 1.1 equiv) after the ice bath drops to 0°C. Trifluoromethanesulfonic acid (20 mmol, 2.0 equiv) was slowly added dropwise under stirring, and the reaction solution changed from t...
Embodiment 1
[0054]
[0055] Mix 1-naphthoic acid (compound II-1, 0.3mmol, 1 equivalent) and palladium acetate (0.03mmol, 10mol% equivalent), add 4 mL of dichloroethane as solvent, and then add diphenyliodonium trifluoromethanesulfonate Salt (compound III-1, 0.6mmol, 2 equivalents), trifluoromethanesulfonic acid (0.09mmol, 30mol% equivalents), reacted for 24 hours at a temperature of 80 ° C, cooled to room temperature, directly spin-dried, dried Separation and purification by column chromatography (eluent: petroleum ether: ethyl acetate = 20:1) to obtain the yellow solid target product, compound I-1, with a yield of 67%. 1 H NMR (400MHz, CDCl 3 )δ8.71(dd, J=7.3,1.0Hz,1H),8.47(dt,J=12.8,6.4Hz,1H),8.36(d,J=7.4Hz,1H),8.26(d,J=8.1 Hz,1H),8.15(d,J=8.0Hz,1H),7.93(d,J=8.1Hz,1H),7.71(ddd,J=13.5,10.2,4.5Hz,2H),7.60(dd,J =15.4,7.5Hz,1H),7.53(dd,J=11.1,3.9Hz,1H). 13 C NMR (101MHz, CDCl 3 )δ 183.91, 136.22, 135.19, 133.42, 133.00, 131.16, 130.26, 129.83, 128.53, 128.33, 128.16, 127.89, 126.83, ...
Embodiment 2
[0057]
[0058] Mix 4-methyl-1-naphthoic acid (compound II-2, 0.3 mmol, 1 equivalent) and palladium acetate (0.03 mmol, 10 mol% equivalent), add 4 mL of dichloroethane as solvent, and then add diphenyl iodide Onium trifluoromethanesulfonate (compound III-2, 0.6mmol, 2 equivalents), trifluoromethanesulfonic acid (0.09mmol, 30mol% equivalents), reacted at a temperature of 80°C for 24 hours, cooled to room temperature, Direct spin-drying, separation and purification by dry loading column chromatography (eluent: petroleum ether: ethyl acetate = 20:1), the target product (compound I-2) was obtained as a yellow solid with a yield of 65%. 1 H NMR (400MHz, CDCl 3 )δ8.58(d, J=7.5Hz, 1H), 8.46(dd, J=7.9, 1.2Hz, 1H), 8.33(d, J=7.4Hz, 1H), 8.24(d, J=8.1Hz, 1H), 8.06(d, J=8.4Hz, 1H), 7.71–7.65(m, 1H), 7.62–7.57(m, 1H), 7.51(dd, J=12.0, 4.1Hz, 2H), 2.78(s ,3H). 13 C NMR (101MHz, CDCl 3 )δ 183.74, 143.30, 136.32, 133.25, 132.05, 131.10, 129.82, 128.22, 128.01, 127.96, 127.88, 127.16, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com