Synthetic method of 17-membered macrocyclic lipopeptide natural compound
A natural compound and synthetic method technology, applied in the field of organic synthesis, can solve problems such as low extraction efficiency and modified compound structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
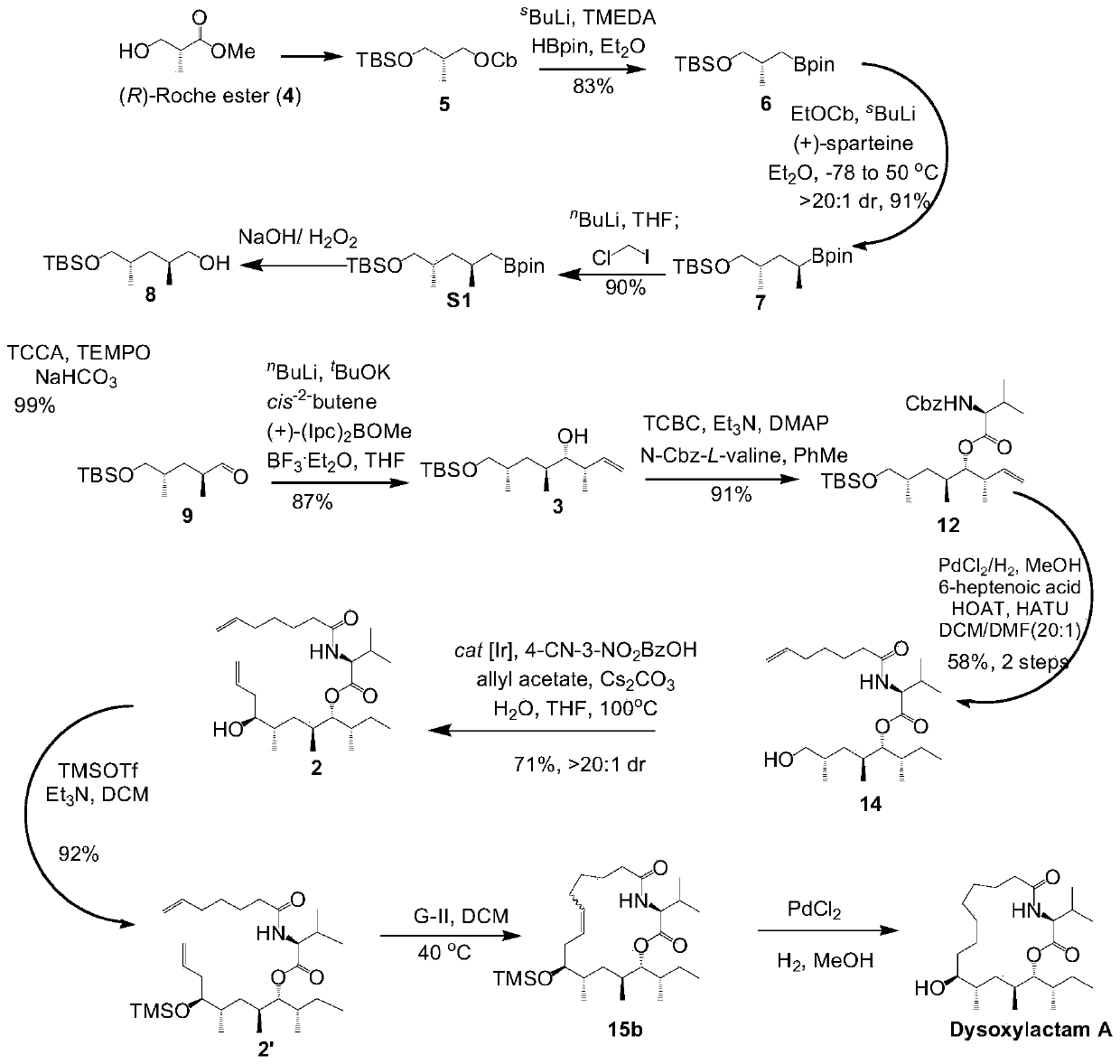
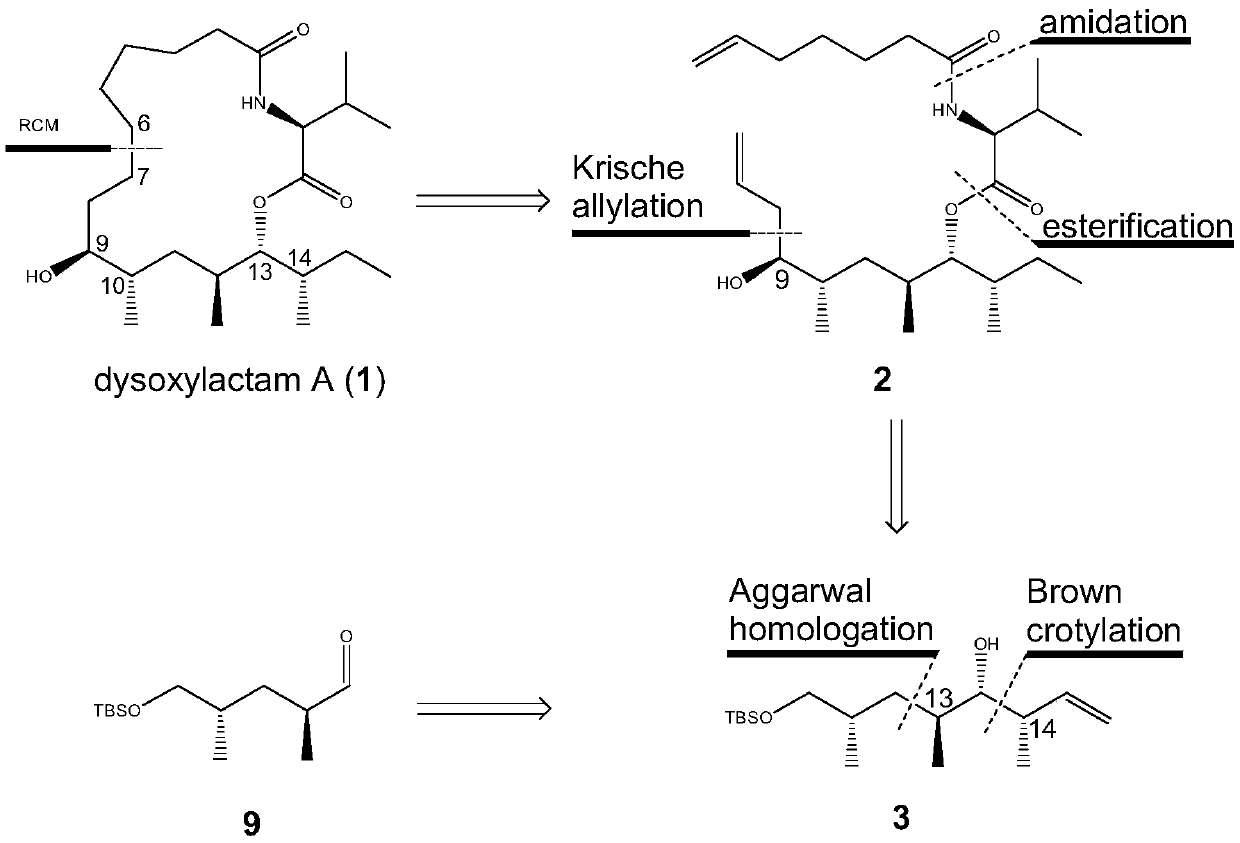
[0020] The embodiment of the present invention provides a synthetic method of a seventeen-membered macrocyclic lipopeptide natural compound, comprising the following steps:
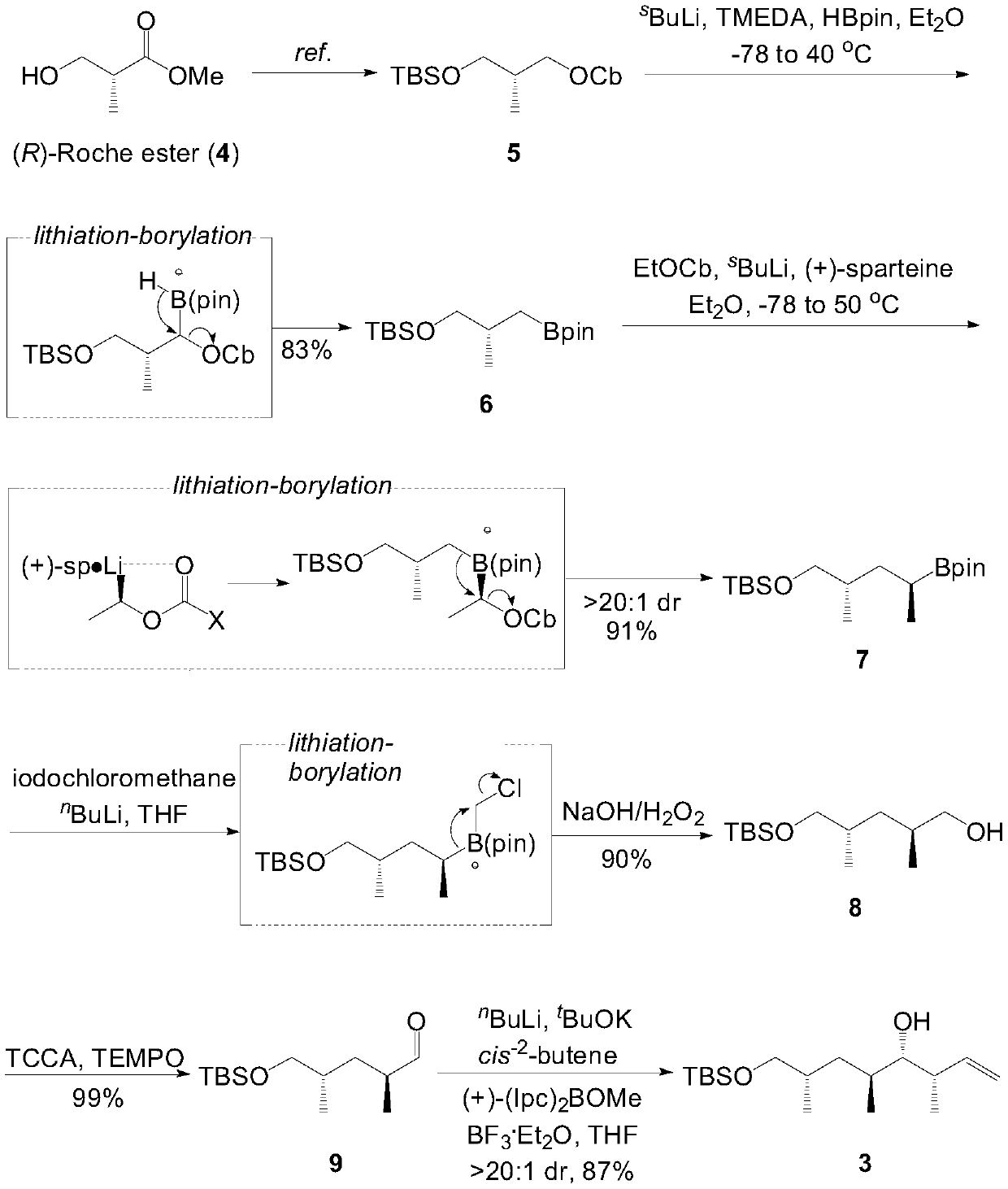
[0021] S00. Obtain (R)-3-hydroxyisobutyrate methyl ester, and sequentially carry out reduction condensation reaction, lithiation-borylation reaction, migration reaction, lithium halide Exchange reaction and oxidation treatment to obtain compound 9;
[0022] S60. Performing an asymmetric Brownylation reaction on the compound 9 to obtain the compound 3;
[0023] S70. Esterifying the compound 3 with aminobenzyloxycarbonyl-protected L-valine to obtain compound 12;
[0024] S80. Catalyzing the hydrogenation reaction of the compound 12 and then reacting with 6-heptenoic acid to obtain the compound 14;
[0025] S90. Performing an allylation reaction on the compound 14 to obtain compound 2;
[0026] S11. Carrying out a ring-closing metathesis reaction on the compound 2 to obtain compound 15;
[0027] S12. Cat...
Embodiment 1
[0097] The steps for preparing compound 6 include: at -40 ° C ~ -80 ° C s BuLi (a solution in hexanes, 1.3M, 1.9mL, 2.48mmol, 2.0eq.) was slowly added dropwise to carbamate 5 (411mg, 1.24mmol, 1.0eq.) and TMEDA (0.41mL, 2.73mmol, 2.2eq .) in anhydrous diethyl ether (15mL, 0.08M). React at -40°C to -80°C for 5 to 8 hours and then slowly dropwise add HBpin (0.54mL, 3.72mmol, 3.0eq.) to a solution of ether (2mL). After reacting at -40°C to -80°C for one to three hours, remove the cold trap, and raise the temperature of the reaction solution to 30°C to 50°C to continue the reaction for 10 to 15 hours. After the reaction is over, cool the reaction solution to 0°C to 25°C and add KH to the system 2 PO 4 Aqueous solution (1M aq., 5 mL) was used to quench the reaction and stirring was continued for 10-30 minutes. The reaction solution was extracted with diethyl ether (3x 10 mL), and the combined organic phases were washed with water (15 mL) and saturated brine (20 mL) successively...
Embodiment 2
[0099] The steps for preparing compound 7 include: at -40 ° C ~ -80 ° C s BuLi (a solution in hexanes, 1.3M, 0.74mL, 0.96mmol, 3.0eq.) was slowly added dropwise to ethyl carbamate (166mg, 0.96mmol, 3.0eq.) and (+)-sparteine (0.29mL, 1.28mmol, 4.0eq.) in anhydrous ether (10mL, 0.1M) solution. React at -40°C to -80°C for 3 to 5 hours and then slowly dropwise add compound 6 (100mg, 0.32mmol, 1.0eq.) to a solution of diethyl ether (5mL). After reacting at -40°C to -80°C for one to three hours, remove the cold trap, raise the temperature of the reaction solution to 30-50°C and continue the reaction for 10-15 hours. After the reaction is over, cool the reaction solution to 0°C to 25°C and add KH to the system 2 PO 4 The reaction was quenched with aqueous solution (1M aq., 5 mL) and stirring was continued for 10 minutes. The reaction solution was extracted with diethyl ether (3x 10 mL), and the combined organic phases were washed with water (15 mL) and saturated brine (20 mL) s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com