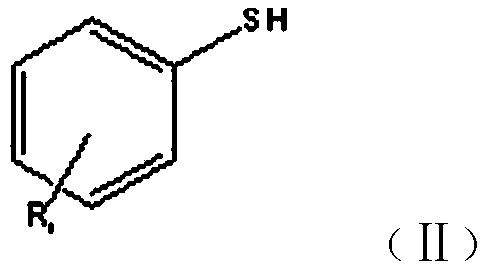
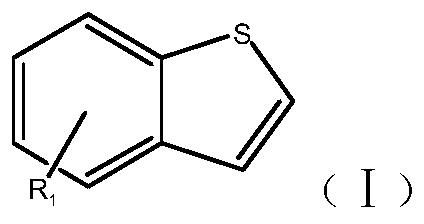
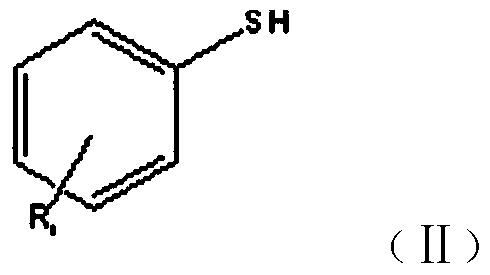
New synthesis method of benzothiacyclopentadiene
A benzothiolene, a newly synthesized technology, applied in the direction of organic chemistry, can solve the problems of harsh reaction conditions, high reaction temperature, low yield, etc., to improve product yield and content, and mild reaction conditions , The effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Synthesis of Benzothiole
[0036] (1) Add 400ml of xylene and 320g of sodium bicarbonate to a 2L four-neck flask, start stirring, start to add 1250g of 10% chloroacetaldehyde dropwise, control the material temperature at 20-25°C with cooling water, take 60 minutes, drop After the addition is complete, stir for 20 minutes, add 150 g of thiophenol into the reaction bottle, after the addition is complete, raise the temperature to 60-65 ° C, keep the temperature constant for 5 hours, after the reaction is completed, separate layers, take the upper organic layer as the intermediate layer, and use it in the next step synthesis;
[0037] (2) Add 800ml of xylene and 50g of polyphosphoric acid to a 2L four-neck flask, stir and raise the temperature to 80°C, add 80g of phosphorus pentoxide, then raise the temperature to 120-125°C, and start to slowly add the step intermediate, At the same time, the temperature of the kettle was controlled at 120-125°C for 5 hours. After the addi...
Embodiment 2
[0039] Synthesis of Benzothiole
[0040] (1) Add 400ml of toluene and 260g of potassium carbonate to a 2L four-neck flask, start stirring, start to add 1250g of 10% chloroacetaldehyde dropwise, use cooling water to control the temperature of the material at 20-25°C, take 60 minutes, and complete the dropwise addition , stirred for 20 minutes, added 150 g of thiophenol into the reaction bottle, after the addition was completed, the temperature was raised to 60-65 ° C, and the temperature was kept constant for 5 hours. After the reaction was completed, the layers were separated, and the upper organic layer was taken as the intermediate layer for the next step of synthesis;
[0041] (2) Add 800ml of toluene and 50g of polyphosphoric acid into a 2L four-neck flask, stir and heat up to 80°C, add 80g of phosphorus pentoxide, then raise the temperature to 120-125°C, and start to slowly add the intermediates dropwise, at the same time Control the temperature of the kettle at 120-125°C...
Embodiment 3
[0043] Synthesis of 5-Chlorobenzothiolene
[0044] (1) Add 200ml of ethylbenzene and 160g of sodium bicarbonate to a 1L four-necked flask, start stirring, start to add 625g of 10% chloroacetaldehyde dropwise, control the temperature of the material at 20-25°C with cooling water, take 40 minutes, drop After the addition is completed, stir for 20 minutes, add 100 g of 4-chlorothiophenol into the reaction bottle, after the addition is completed, heat up to 65-70 ° C, keep the temperature constant for 4 hours, after the reaction is completed, separate layers, take the upper organic layer as the intermediate layer, and use synthesized in the next step;
[0045] (2) Add 600ml of ethylbenzene and 50g of polyphosphoric acid to a 2L four-necked flask, stir and raise the temperature to 80°C, add 50g of phosphorus pentoxide, then raise the temperature to 125-130°C, and start adding the step intermediate slowly, At the same time, the temperature of the kettle was controlled at 125-130°C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com