Chlorin e6 ferrocene conjugate with photo- and acoustic-sensitive activity as well as preparation method and application
A technology of chlorin and ferrocene, which is applied in the field of chemical medicine and can solve the problems of large required dose, low generation of active oxygen, slow removal rate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
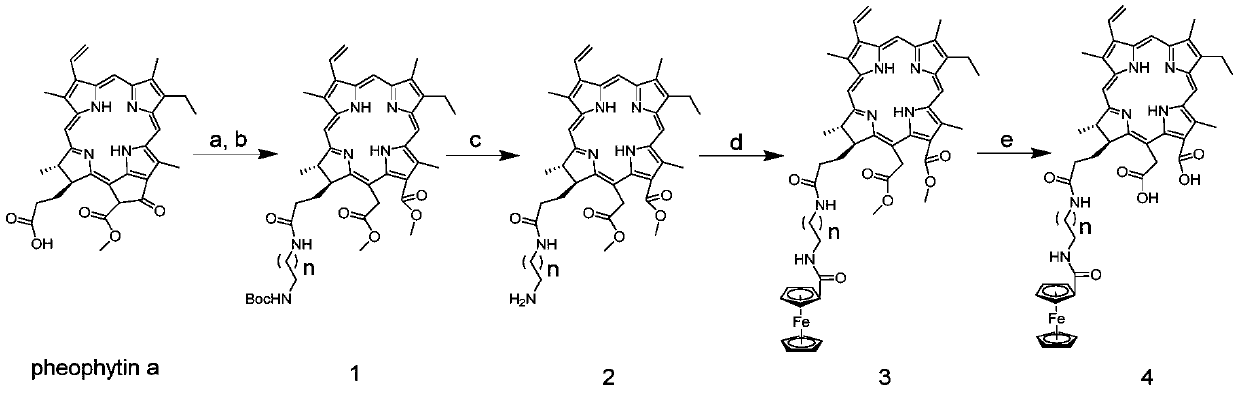
[0040] Synthesis of Intermediate N-Succinimidyl Ferrocenecarboxylate
[0041] Dissolve Ferrocenecarboxylic acid (1.003g) in CH 2 Cl 2 (15mL), at 0 ℃, add EDCI (1.303g), N-Hydroxy succinimide (1.001g), at room temperature under N 2 React in the environment for 10h, after the reaction is completed in CH 2 Cl 2 :H 2 Extraction and removal of excess reactants and by-products in O solution system, anhydrous NaSO 4 dry. The target compound was purified in a 200-300-mesh silica gel column using a dichloromethane elution system to obtain 1.228 g, with a yield of 86%. 1 H-NMR (CDCl 3 ,400MHz,ppm)δ:4.94(t, J=4.0Hz,2H),4.57(t,J=4.0Hz,2H),5.00(s,5H),4.29(d,J=8.0Hz,4H). HR-MS found: m / z: 350.0079[M+Na] + , calcd for C 15 h 13 FeNO 4 ,327.0194.
[0042] Synthesis of compound 1
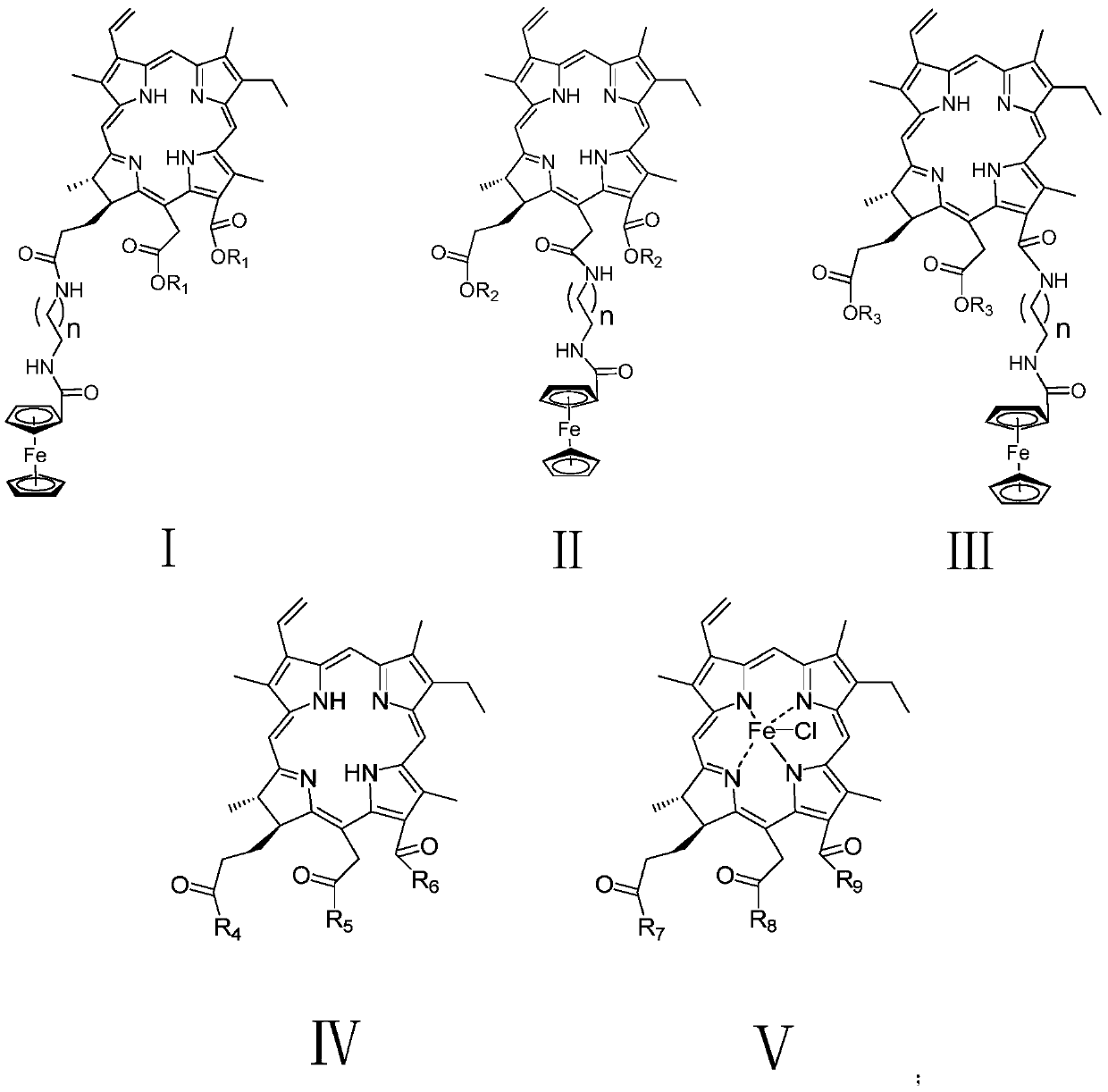
[0043] Pheophorbide a (3017.2 mg) with a purity of 51% was dissolved in DMF (35 mL), and N-Boc-ethylenediamine (980.5 mg) and EDCI (1165.0 mg) were added to the reaction system at 0° C. to room temper...
Embodiment 2
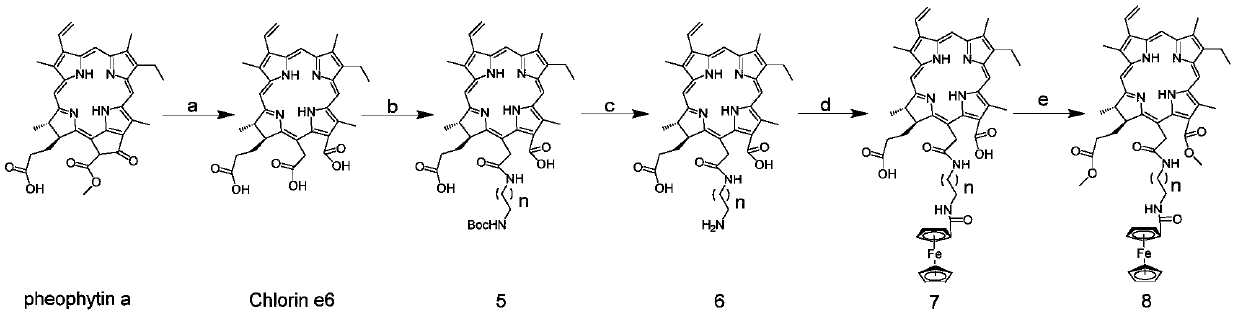
[0051] Synthesis of Chlorin e6
[0052] Pheophorbide a (300 mg) with a purity of 51% was dissolved in THF (7.5 mL), and an aqueous potassium hydroxide solution (4 mL, 5%) was added to the reaction system at 0° C., and then slowly rose to room temperature, and N 2 Protected and stirred overnight. TLC detects the progress of the reaction. After the reaction was complete, THF was evaporated under reduced pressure, diluted with water, and the pH of the diluted solution was adjusted to 3 with hydrochloric acid (2 mol / L), and the substance was precipitated in the aqueous solution. The precipitate was filtered, washed, and dried in vacuum. Not purified by column chromatography. 1 H-NMR (DMSO-d 6 ,400MHz,ppm)δ:9.73 (s,1H,10-H),9.59(s,1H,5-H),9.11(s,1H,20-H),8.12(dd,1H,J=16.0, 16.0Hz, 3 1 -H),6.35(d,1H,J=16.0Hz,3 2a -H),6.11(d,1H,J=12.0Hz,3 2b -H),5.40~5.48(m,2H,15 1 -H), 4.65(d, 1H, J=8.0Hz), 4.48(d, 1H, J=8.0Hz), 3.69~3.71(m, 2H, 8 1 -H),3.60(s,3H,12 1 -CH 3 ),3.64(s,3H,1...
Embodiment 3
[0062] Synthesis of compound 9
[0063] Pheophorbide a (42.3 mg) with a purity of 51% was dissolved in sulfuric acid methanol solution (0.1 g of sulfuric acid + 1.9 g of methanol) at 0°C. Then slowly warmed up to room temperature, N 2 Protected and stirred overnight. TLC detects the progress of the reaction. After the reaction is completed in CH 2 Cl 2 : saturated NaHCO 3 Extraction and removal of excess reactants and by-products in aqueous solution system, anhydrous NaSO 4 dry. The target compound was purified in a 200-300 mesh silica gel column using dichloromethane: methanol (60:1) + 1% formic acid elution system to obtain 18.4 mg, with a yield of 83.3%.
[0064] Synthesis of Compound 10
[0065] Compound 9 (163.8 mg) was dissolved in CH 2 Cl 2 (10mL), added ethylenediamine (1mL) to the reaction system at 0°C, then slowly warmed up to room temperature, N 2 Protected and stirred overnight. TLC detects the progress of the reaction. Ethylenediamine was removed by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com