Modified duck circovirus Cap protein and preparation method and application thereof
A technology of duck circovirus and protein, applied in the direction of antiviral immunoglobulin, biochemical equipment and methods, viruses, etc., can solve the problems of limited application and inactivity, and achieve the promotion of immunogenicity, excellent effect and good application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of Transformed Cap Protein of Duck Circovirus and Obtainment of Engineering Bacteria
[0023] 1. DNAStar biological software analyzed the Cap protein sequence of duck circovirus SDFC12 strain (GenBank accession number: KY328304), and found that its N-terminal 21-36 peptide segment (RRFRRRRLRIARPRRR) is rich in arginine, which will seriously affect its ability in Escherichia coli Recombinant expression in expression systems. At the same time, the peptide of Yersinia pestis (TAKSKKFPSYTATYQF) is a T cell epitope with good cellular immune function, which can significantly improve the cellular immune function of various immunogens. Therefore, the present invention replaces the arginine-rich N-terminal 21-36 peptide of duck circovirus Cap protein with a T cell epitope peptide (TAKSKKFPSYTATYQF) to obtain a modified duck circovirus Cap protein, At the same time, in order to facilitate the nickel column purification of the subsequent modified protein, 6 histidines...
Embodiment 2
[0032] Preparation and Testing of Genetic Engineering Subunit Vaccine of Duck Circovirus
[0033] 1 bacterial strain
[0034] 1.1 The strain used for production is duck circovirus genetically engineered subunit vaccine production strain DP.
[0035] 1.2 Standards for strains used in production
[0036] 1.2.1 Morphological and biochemical properties
[0037]Cultivate overnight on the LB agar plate containing kanamycin, round, smooth colonies with neat edges, protrusions, milky white luster appear on the culture plate, and after Gram staining, they are Gram-negative short bacilli under the microscope; The biochemical test results were glucose fermentation+, indole test+, methyl red test+, VP-, citric acid utilization test-.
[0038] 1.2.2 Culture characteristics It can grow in the medium containing kanamycin.
[0039] 1.2.3 Identification test
[0040] 1.2.3.1 PCR detection Use the LB liquid culture of this bacteria as a template, and use the following PCR primers for PCR a...
Embodiment 3
[0077] Efficacy Test of Genetic Engineering Subunit Vaccine of Duck Circovirus
[0078] 1 Preparation of control vaccine
[0079] Referring to the preparation procedure of tissue-inactivated vaccines in the 2015 edition of "Chinese Veterinary Pharmacopoeia", duck circovirus WF strain was added in proportion to 10% formaldehyde solution, the final concentration of formaldehyde solution was 0.2%, and inactivated at 37°C for 12 hours. After the inactivation is complete, prepare duck circovirus tissue inactivated vaccines according to the method of 2.5 vaccine preparation part and 3 finished product inspection part in embodiment 2.
[0080] 2 Animal efficacy experiment
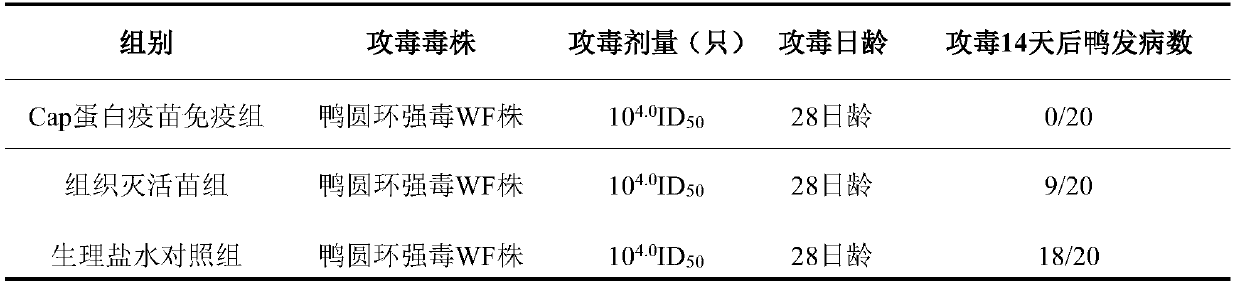
[0081] Sixty 7-day-old healthy susceptible ducks were equally divided into two groups, 20 in each group. The first group is the Cap protein vaccine immunization group, and the duck circovirus genetically engineered subunit vaccine prepared by the present invention is used for leg muscle immunization, 0.2ml / head....
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com