Synthesis method and application of miRNA-carrying VB12 binding type nano-composite
A technology of nanocomposites and synthesis methods, which is applied in the field of synthesis of nanomedicines, can solve problems such as poor stability of VB12, and achieve the effects of convenient operation, low toxicity and anti-tumor effect, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] In some examples, the synthesis method of the PLGA-PEG nanocarrier comprises:
[0041] 1) Mix PLGA, NHS and EDC in anhydrous solvent B, activate, and after full reaction, wash with anhydrous solvent C to remove unreacted small molecules, and dry to obtain PLGA-NHS pellets;
[0042] 2) Dissolve PLGA-NHS pellets in anhydrous solvent D, add bifunctional modified NH 2 - PEG-COOH and diisopropylethylamine, continuous reaction for 24 hours;
[0043] 3) After the reaction is finished, wash with anhydrous solvent C, and finally vacuum-dry to obtain the PLGA-PEG nanocarrier.
[0044] In some embodiments, a method for synthesizing vitamin B12-binding nanocarriers includes:
[0045] 1) Mixing and dissolving PLGA-PEG nanocarriers, vitamin B12, EDC and DMAP in anhydrous solvent A, stirring and reacting at 15-40°C;
[0046]2) After the reaction is completed, dialyze and remove the solvent to obtain a vitamin B12-binding nanocarrier, which is denoted as VB12-PLGA-PEG nanocarrier. ...
Embodiment 1
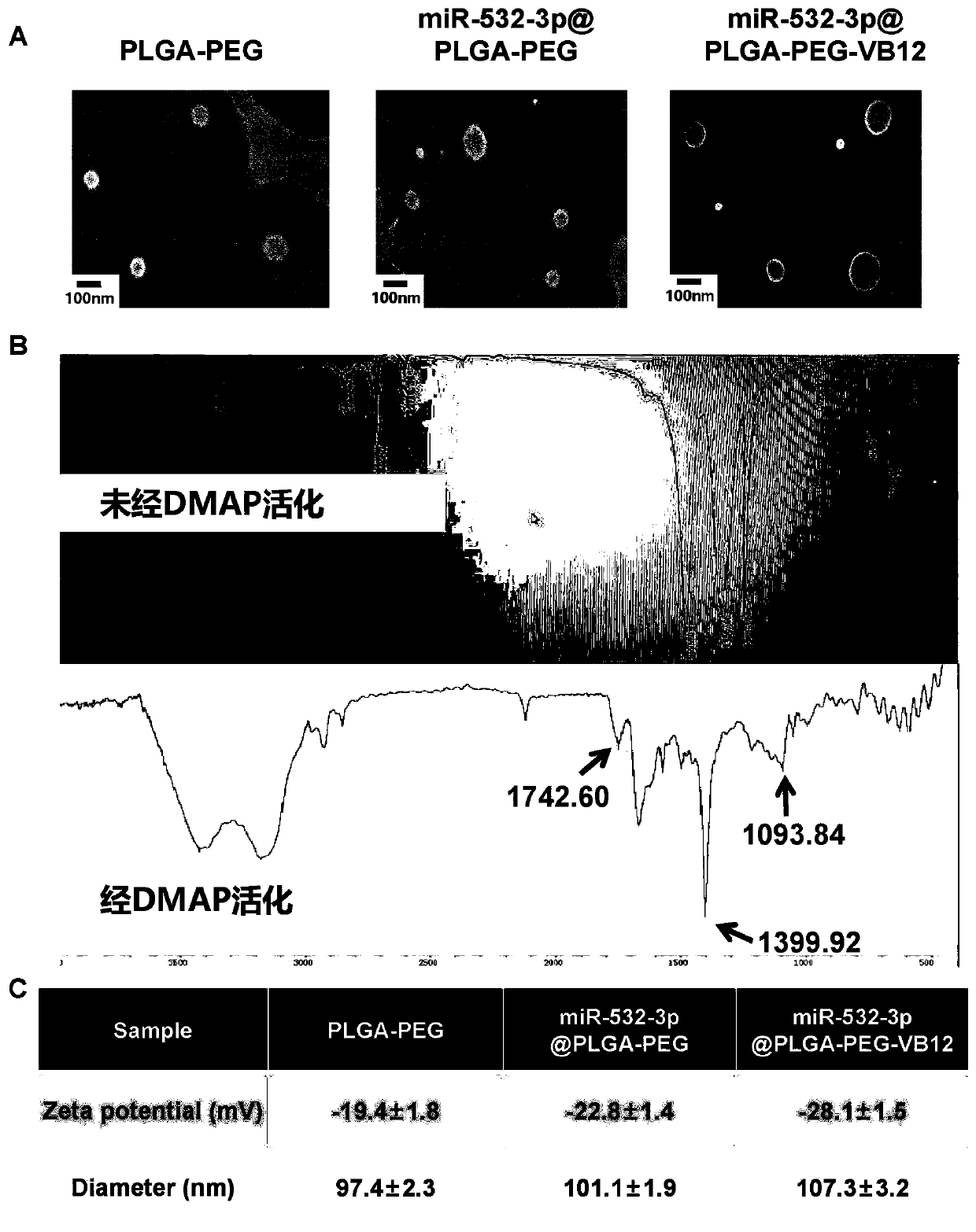
[0077] Example 1: Synthesis and physical and chemical characterization of miR-532-3p@PLGA-PEG-VB12 nanocomposite
[0078] (1). Material synthesis:
[0079] 1) Synthesis of PLGA-PEG-COOH derivatives: First, 250 mg of PLGA solution was mixed with NHS and EDC in dry dichloromethane (CH 2 Cl 2 ) conditions; after 4 hours, the mixture was collected and washed with pre-cooled MeOH / Et 2 O(1:1) washed twice, and then dried in a vacuum environment; subsequently, the dried PLGA-NHS pellets were dissolved in dry chloroform, and the bifunctional modified NH 2 -PEG-COOH and diisopropylethylamine were reacted; after 24 hours, MeOH / Et 2 O (1:1) washing, and vacuum drying to obtain PLGA-PEG-COOH complex;
[0080] 2) Preparation of VB12-labeled PLGA-PEG nanocomposites: PLGA-PEG nanocomposites (1mol), vitamin B12 (1mol) and EDC (1.1mol) obtained in step ① were added to the mixture containing or not containing DMAP (0.1mol) Butylene oxide solution, stirred at room temperature for 4 hours. ...
Embodiment 2
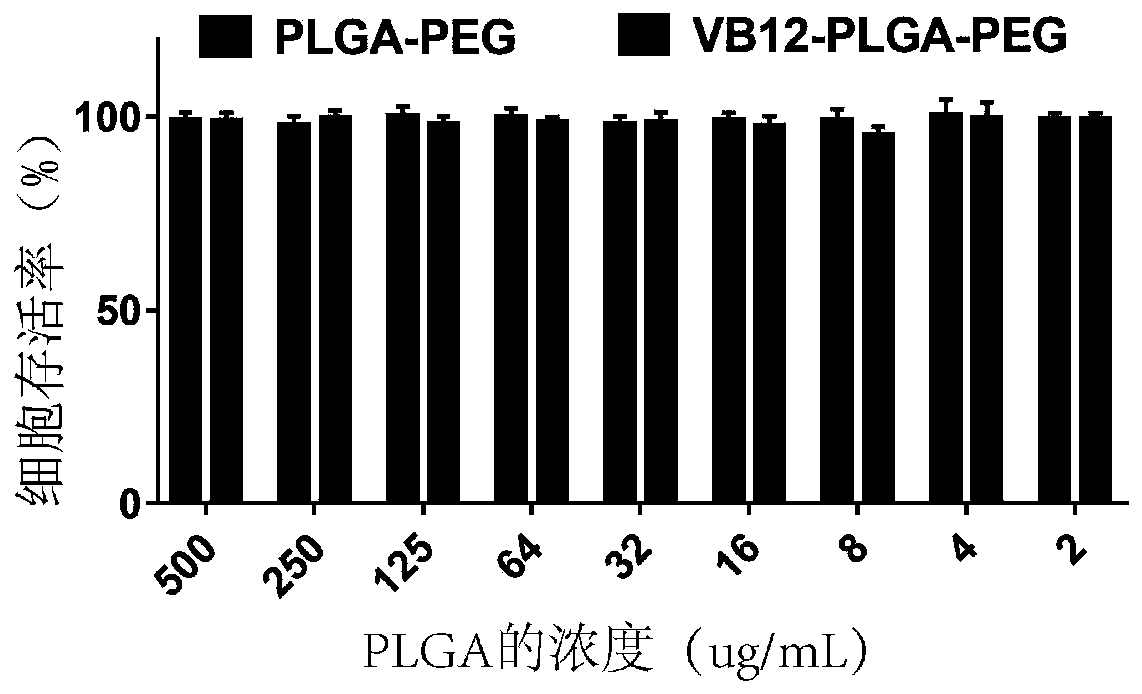
[0089] Embodiment 2: the safety of VB12-PLGA-PEG nano carrier
[0090] After co-incubating different concentrations of PLGA-PEG and PLGA-PEG-VB12 nanocarriers (2-500ug / mL) with gastric normal mucosal epithelial cells (GES-1) for 24 hours, the Cell Counting Kit (CCK-8) was used to experiment Detection of cell viability.
[0091] figure 2 is the safety result of miR-532-3p@PLGA-PEG-VB12 nanocomplex. The results of CCK-8 showed that under the treatment of PLGA-PEG and VB12-PLGA-PEG nanocarriers at multiple concentrations in the range of 2-500 μg / mL, the cell survival rate of GES-1 cells was above 90% ( figure 2 ), so PLGA-PEG and VB12-PLGA-PEG nanocarriers are relatively safe nanomaterials.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com