Application of chimonanthus salicifolius ethyl acetate parts in preparation of auxiliary anti-tumor drugs
An anti-tumor drug, the technology of ethyl acetate, applied in the field of medicine, can solve problems such as organ side effects and toxic tissues, and achieve the effects of alleviating weight loss, protecting integrity, and reducing liver toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
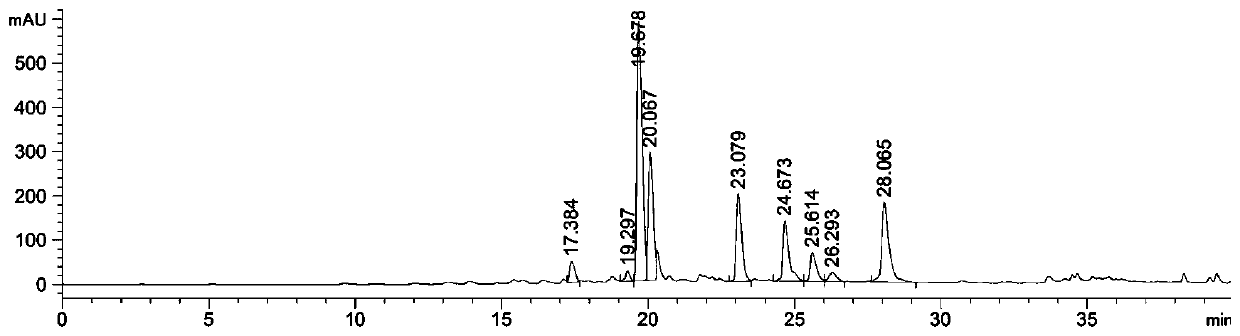
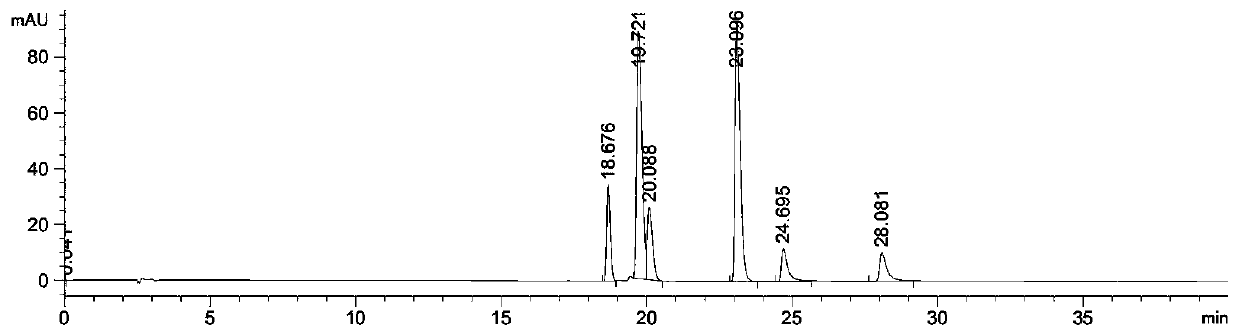
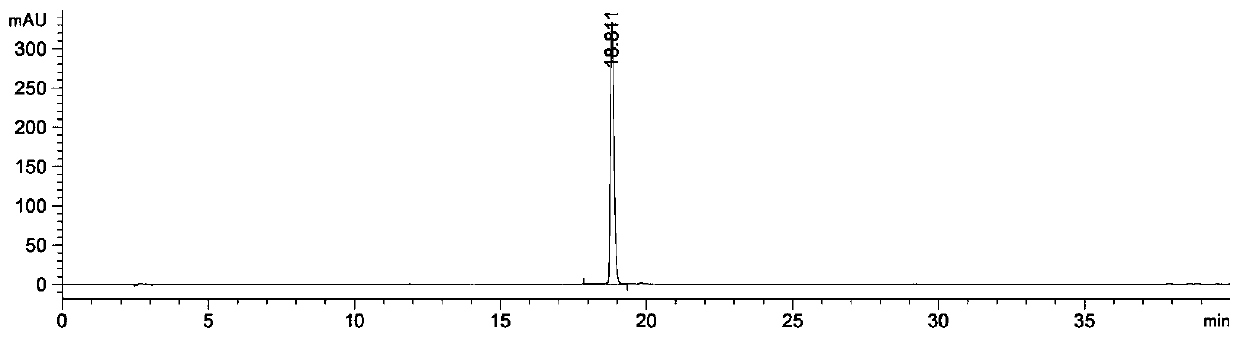
Image
Examples
Embodiment 1
[0031] Preparation of the ethyl acetate part of Chimerus osmanthus: Weigh 2000 g of the stems and leaves of Wintersweet willow that has been crushed and sieved, add 30 L of 90% ethanol aqueous solution to soak overnight, then heat and reflux with 90% ethanol aqueous solution to extract 2 times, each time 2h, suction filtration, combined filtrates to obtain the first filtrate, then reflux extraction with 16L volume fraction of 60% ethanol aqueous solution 3 times, each time 2h, suction filtration, combined filtrates to obtain the second filtrate, combined first filtrate and the second filtrate were concentrated under reduced pressure to obtain an extract; the obtained extract was dispersed in water, and then extracted with petroleum ether, chloroform, and ethyl acetate in sequence to finally obtain the ethyl acetate fraction of Waxwort. The ethyl acetate part of the willow-leaf wintersweet is dissolved in an aqueous solution with a mass fraction of 2% Tween 80, configured to a c...
Embodiment 2
[0033] Embodiment 2 pharmacological experiment
[0034] 50 ICR mice were randomly divided into 5 groups: normal control group, model group, and model + willow-leaved wintersweet group, in which the concentrations of ethyl acetate in willow-leaved wintersweet were 0.30 g / ml, 0.60 g / ml, 0.90 g / ml. After one week of adaptation to a normal diet, all the mice were administered intragastrically for 7 days, once a day, each time at 0.1ml / 10g; among them, the model+Waterweed group were given 3 concentrations of ethyl acetate parts of Willowsweet, The concentrations of the ethyl acetate parts of Waxwort willow were 0.30g / ml (low dose), 0.60g / ml (medium dose), and 0.90g / ml (high dose); the normal control group and the model group were given equal volumes of physiological brine. Modeling was established while intragastric administration, and intestinal mucositis model mice were induced by intraperitoneal injection of 25 mg / kg of 5-Fu injection on days 1 to 7.
[0035] During the exper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com