Novel application of betulin derivatives in preparation of drugs for repairing nerve injury
A nerve injury and new application technology, applied in the field of medicine, can solve the problems of incomplete chemical and pharmacological research on betulin derivatives, lack of active groups in the mother nucleus, and few reaction sites, so as to achieve good nerve cell damage repair and protection Activity, enhance the effect of learning and memory ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
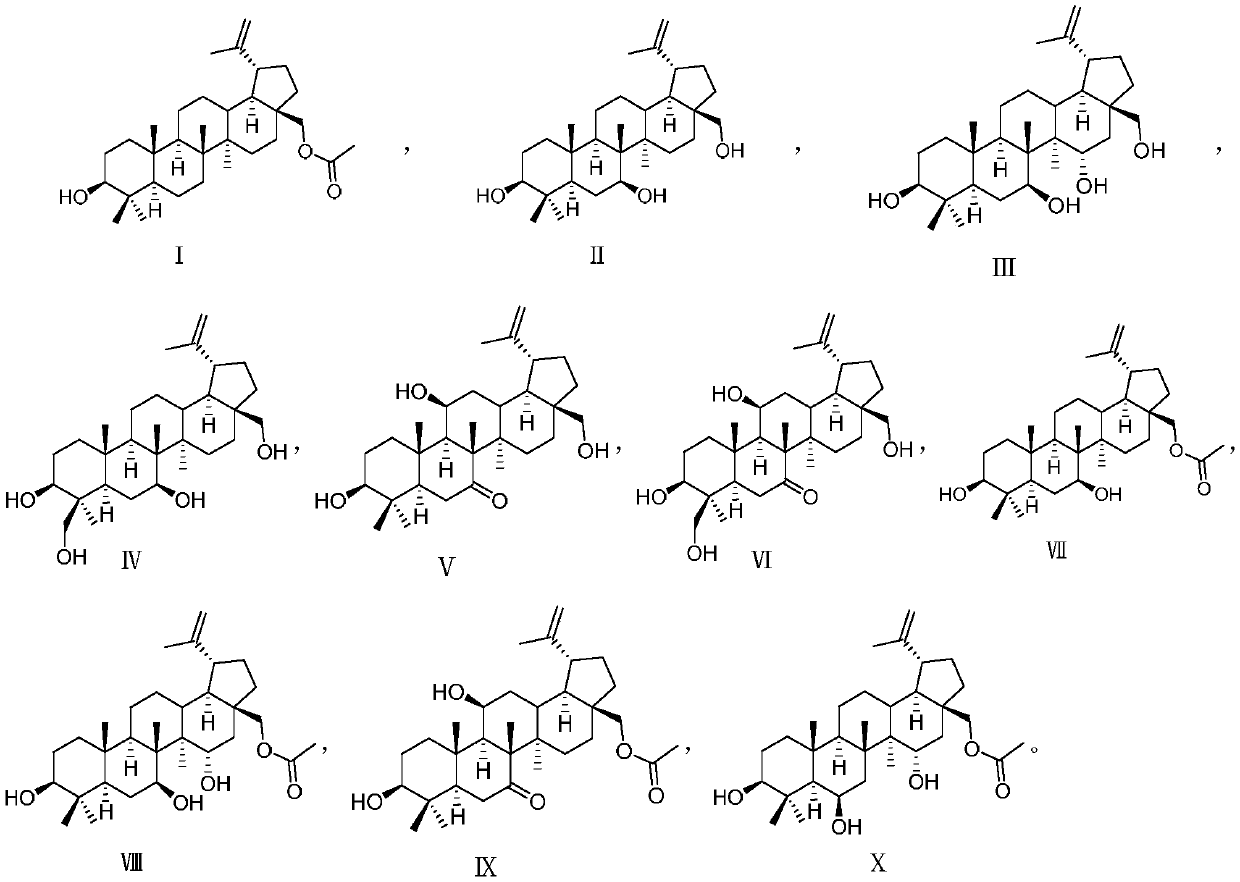
[0021] Example 1 Compound I-X has repairing and protective activity on nerve cells damaged by hydrogen peroxide
[0022] (1) Experimental materials
[0023] CO 2 Incubator (Jouan IGO150); Microplate reader (Bio-TEK ELx800); Fluorescent inverted microscope (OlympusIX51); MTT cell proliferation and cytotoxicity detection kit (Biotech Institute), DMEM high glucose medium (Gibcol BRL ), fetal bovine serum, dimethyl sulfoxide (DMSO), trypsin (Shanghai Bioengineering Co., Ltd.), 30% hydrogen peroxide (H 2 o 2 ) (Tianjin Ruijinte Chemicals Co., Ltd.), SH-SY5Y cells (Tumor Institute, Chinese Academy of Medical Sciences).
[0024] (2) Experimental method
[0025] The MTT method was used to determine the H of each test compound 2 o 2 Influence of damaged SH-SY5Y cell viability: count the cells after digestion with trypsin, adjust the cell density of the cell suspension to 5×10 4 cells / mL, add 200 μL to each well of a 96-well culture plate, place in 5% CO 2 , 37°C constant temper...
Embodiment 2
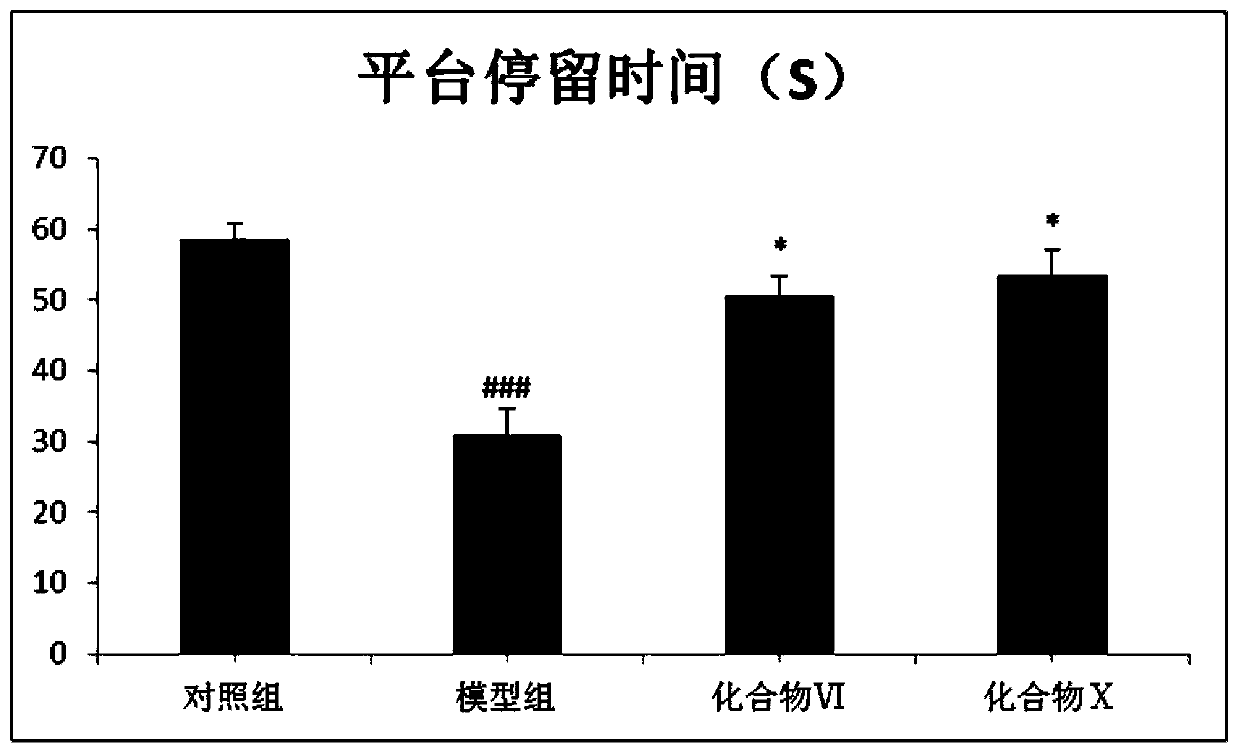
[0031] Example 2 Effect of compound VI and compound X of the present invention on learning and memory ability of aging mouse model
[0032] (1) Establishment of aging mouse model
[0033] Forty male ICR mice of SPF grade, weighing 22-26 g, were randomly divided into 4 groups, namely control group, model group, compound VI group and compound X group. Except for the control group given normal saline, the mice in the other groups were intraperitoneally injected with D-galactose at a dose of 250 mg / kg, once a day, and the drug group was given 10 mg / kg of the compound by intragastric administration, and the control group and the model group were given intragastric administration, etc. Volumetric saline was administered once daily for 6 weeks. Mice were used for later experiments after showing obvious aging characteristics.
[0034] (2) Experimental method
[0035] The water maze laboratory is an experiment in which experimental animals are forced to swim to find a platform hidde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com