Nitrogen-sulfur fused-heterocycle hexacene compound and preparation method and application thereof
A compound, nitrogen and sulfur technology, applied in the field of organic photoelectric small molecule semiconductor materials, can solve the problems of poor solubility, little research, poor stability, etc., and achieve good stability, good solubility, and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
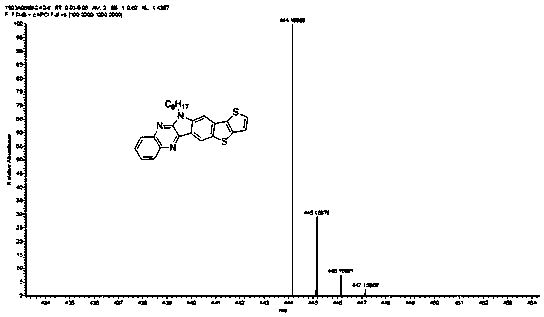
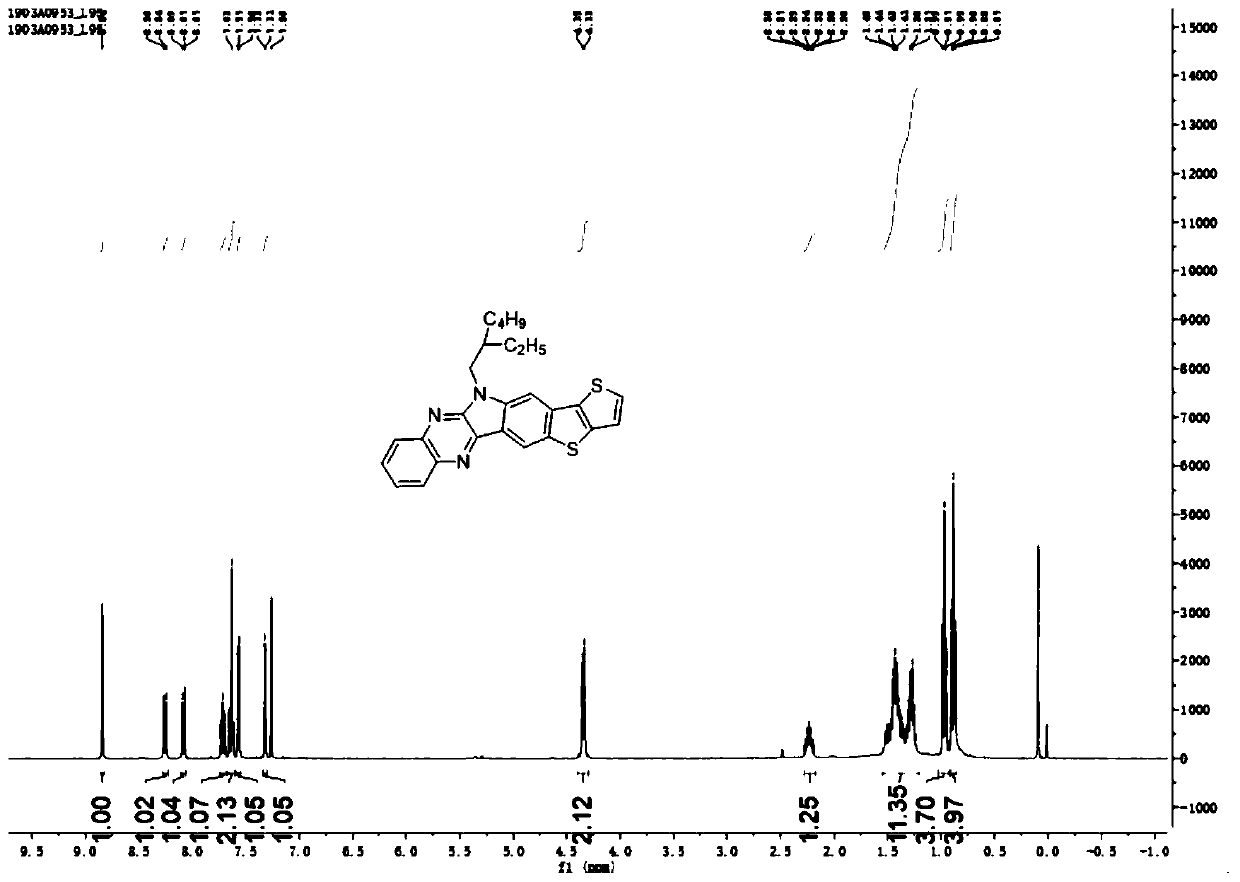
[0063] Embodiment 1 A kind of preparation of nitrogen thia hexacene compound
[0064] 1. Preparation method
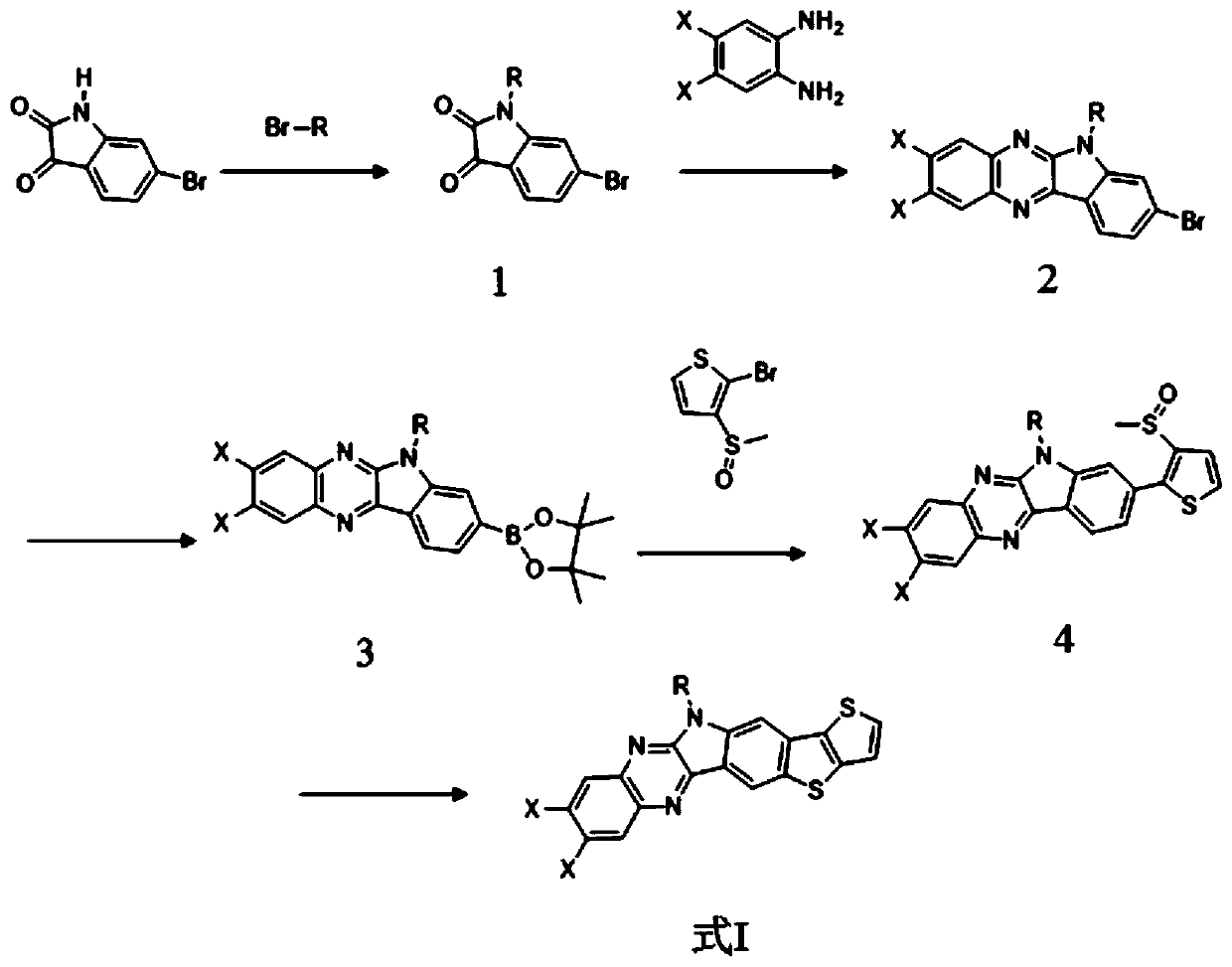
[0065] (1) Under a nitrogen atmosphere, add 2g bromoisatin and 2.5g dried potassium carbonate into a pear-shaped reaction flask, then add 15mL N,N-dimethylformamide (DMF), stir, and then add 2g Bromo with isooctane, react at 80°C for 12h. After the reaction was completed, the reactant was distilled off under reduced pressure to remove the solvent, and then separated and purified by column chromatography. The ratio of eluent dichloromethane to petroleum ether was 1:1, and compound 1' could be obtained by distillation under reduced pressure. The mass of compound II obtained was 2.7 g, and the yield was 90%;
[0066] (2) Under a nitrogen atmosphere, add 1 g of bromoisatin (1') connected to isooctane chains and 383 mg of 1,2-phenylenediamine into a single-necked flask, then add 8 mL of glacial acetic acid as a catalyst and heat at 115 ° C Reflux, reaction 10h. The obta...
Embodiment 2
[0074] Embodiment 2 Preparation of azathiahexacene compound
[0075] 1. Preparation method
[0076] (1) Under a nitrogen atmosphere, add 2g bromoisatin and 2.5g dried potassium carbonate into a pear-shaped reaction flask, then add 15mL N,N-dimethylformamide (DMF), stir, and then add 2g Bromine with n-octane reacted at 80°C for 12h. After the reaction is completed, the reactants are distilled under reduced pressure to remove the solvent, and then separated and purified by column chromatography. The ratio of eluent dichloromethane and petroleum ether is 1:1, and compound 1" can be obtained by distillation under reduced pressure. Compound The quality of is 2.7g, and the productive rate is 90%;
[0077] (2) Under a nitrogen atmosphere, add 1 g of bromoisatin (1") connected with n-octane chains, and 383 mg of 1, 2-phenylenediamine into a single-necked flask, then add 8 mL of glacial acetic acid as a catalyst and heat to reflux, Reaction for 12 hours. The obtained reactant was di...
Embodiment 3
[0085] Embodiment 3 Preparation of azathiahexacene compound
[0086] 1. Preparation method
[0087] (1) Under a nitrogen atmosphere, add 2g bromoisatin and 2.5g dried potassium carbonate into a pear-shaped reaction flask, then add 15mL N,N-dimethylformamide (DMF), stir, and then add 2g Bromine with n-octane reacted at 80°C for 12h. After the reaction was completed, the reactant was distilled off under reduced pressure to remove the solvent, and then separated and purified by column chromatography. The ratio of eluent dichloromethane to petroleum ether was 1:1, and compound 1"' could be obtained by distillation under reduced pressure. The quality of the compound is 2.7g, and the yield is 90%;
[0088](2) Under a nitrogen atmosphere, add 1 g of bromoisatin (1") connected to n-octane chains and 523 mg of 4,5 dichloro-1,2 phenylenediamine into a single-necked flask, and then add 8 mL of glacial acetic acid As a catalyst, it is heated to reflux and reacted for 12 hours. The obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com