Method for preparing 4-chloro-3, 5-dimethylphenol
A technology of dimethylphenol and alkyl, which is applied in the field of organic chemical synthesis, can solve the problems of troublesome post-treatment of three wastes, low para-selectivity, heterogeneous reaction, etc., and achieve improved chlorination efficiency and para-selectivity High performance, the effect of improving para-selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
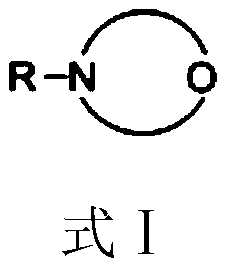
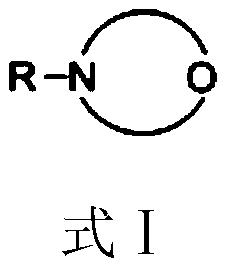
[0038] 122.16 g (1.0 mol) of 3,5-dimethylphenol, 3.05 g (2.5 wt% of 3,5-dimethylphenol) of N-methylmorpholine were dissolved in 91.62 g of tetrachloroethylene to give 3, 5-dimethylphenol solution;
[0039] Add 91.62g of tetrachlorethylene solvent into a 1L stainless steel reaction kettle equipped with a mechanical stirrer, a thermometer, and a gas line pipe connected to a tail gas separation and recovery device, replace it with a nitrogen atmosphere, start stirring and heat up to 100°C; 296.76g (3.0 mol) phosgene and 3,5-dimethylphenol solution were slowly and uniformly fed into the reactor within 0.5 h. After the feeding was completed, the reaction was carried out at a constant temperature of 100°C for 2 h, and the reaction was stopped.
[0040] Cool the reaction liquid to room temperature to crystallize and filter out the crystals to obtain 4-chloro-3,5-dimethylphenol.
[0041] According to GC, the conversion rate of 3,5-dimethylphenol in the reaction solution was 97.8%, th...
Embodiment 2
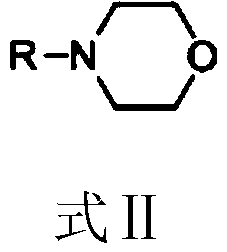
[0043]122.16 g (1.0 mol) of 3,5-dimethylphenol, 2.44 g (2.0 wt% of 3,5-dimethylphenol) of 4-cyclohexylmorpholine were dissolved in 122.16 g of tetrachloroethylene to give 3, 5-dimethylphenol solution;
[0044] Add 122.16g of tetrachlorethylene solvent into a 1L stainless steel reaction kettle equipped with a mechanical stirrer, a thermometer, and a gas line pipe connected to a tail gas separation and recovery device, replace it with a nitrogen atmosphere, start stirring and raise the temperature to 80°C, and 227.52g (2.3 mol) phosgene and 3,5-dimethylphenol solution were slowly and uniformly fed into the reactor within 0.5 h. After the feeding was completed, the reaction was carried out at a constant temperature of 80°C for 2 h, and the reaction was stopped.
[0045] Cool the reaction liquid to room temperature to crystallize and filter out the crystals to obtain 4-chloro-3,5-dimethylphenol.
[0046] According to GC, the conversion rate of 3,5-dimethylphenol in the reaction l...
Embodiment 3
[0048] 122.16g (1.0mol) 3,5-xylenol, 1.22g (1.0wt% of 3,5-xylenol) 4-(2-methoxyethyl)morpholine were dissolved in 152.7g Tetra In vinyl chloride, 3,5-dimethylphenol solution was obtained;
[0049] Add 152.7g of tetrachlorethylene solvent into a 1L stainless steel reaction kettle equipped with a mechanical stirrer, a thermometer, and a gas line pipe connected to a tail gas separation and recovery device, replace it with a nitrogen atmosphere, start stirring and heat up to 85°C, and 197.84g (2.0 mol) phosgene and 3,5-dimethylphenol solution were slowly and uniformly fed into the reactor within 1 hour. After the feeding was completed, react at a constant temperature of 85°C for 3 hours and stop the reaction.
[0050] Cool the reaction liquid to room temperature to crystallize and filter out the crystals to obtain 4-chloro-3,5-dimethylphenol.
[0051] According to GC, the conversion rate of 3,5-dimethylphenol in the reaction liquid was 98.3%, the selectivity of 4-chloro-3,5-dimet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com