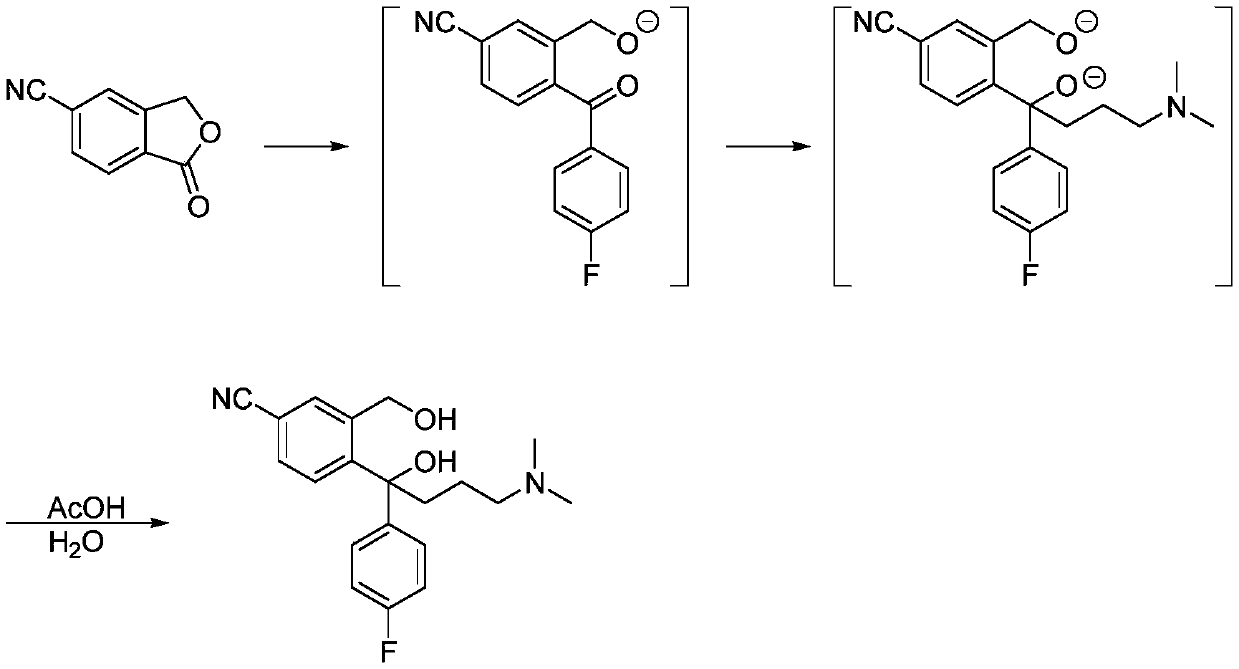
Synthesis method of citalopram intermediate
A technology for citalopram and intermediates, applied in the field of preparation of citalopram intermediate 4--1--1-hydroxybutyl)-3-benzonitrile, can solve the problem of high overall cost and unfavorable industrial production , low yield and other problems, to achieve the effect of strong operability, short synthetic route, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
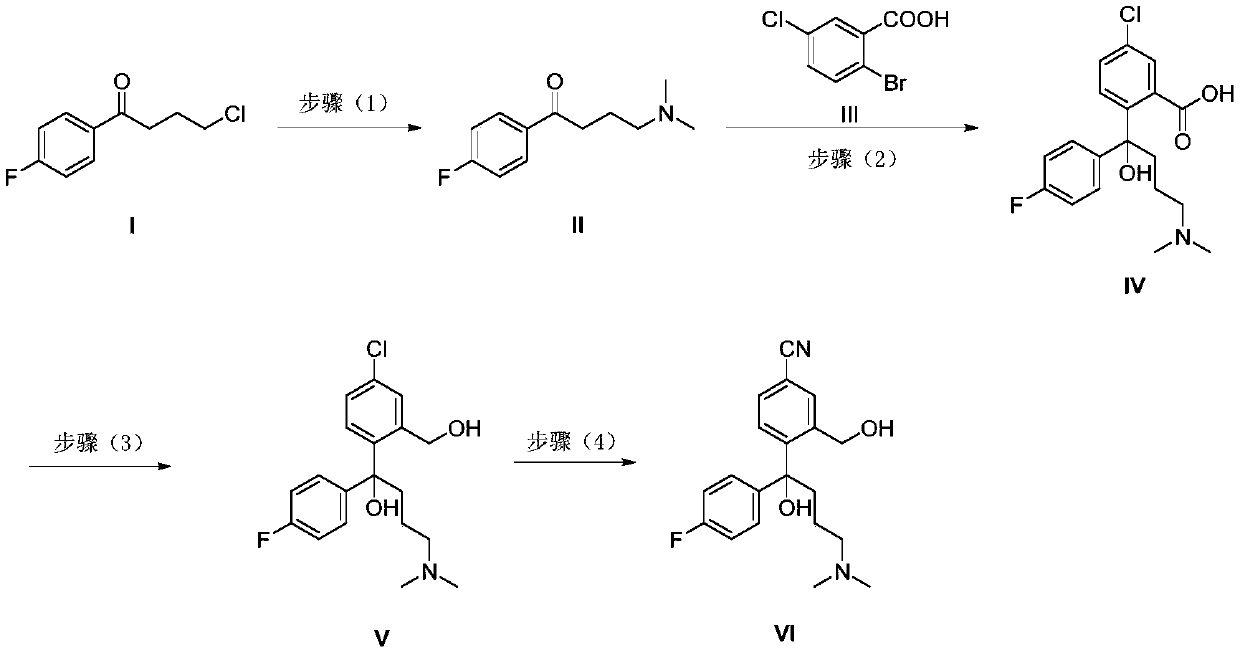
[0041] Example 1 Synthesis of 4-(dimethylamino)-1-(4-fluorophenyl)butan-1-one hydrochloride
[0042] Add 4-chloro-1-(4-fluorophenyl)butan-1-one (20.1g, 0.1 mmol), dimethylamine (40% aqueous solution, 20mL) and ethanol (100mL) into a 250mL three-necked flask in sequence . The reaction was refluxed for 16 h, cooled to room temperature, concentrated under reduced pressure, then added water, and extracted with ethyl acetate. The organic phase was washed again with water, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain a crude product. The crude product was dissolved in acetone, fed with hydrogen chloride gas, stirred for 1 h, and then filtered with suction to obtain the hydrochloride of formula II (21.3 g, 86.9%).
Embodiment 2
[0043] Example 2 Synthesis of 4-(dimethylamino)-1-(4-fluorophenyl)butane-1-one
[0044] Add 4-chloro-1-(4-fluorophenyl)butan-1-one (20.1g, 0.1 mmol), dimethylamine hydrochloride (16.3g, 0.2mol), carbonic acid Potassium (27.6 g, 0.2 mol) and DMF (100 mL). Reacted at 80°C for 16h, cooled to room temperature, added saturated brine, and extracted with ethyl acetate. The organic phase was washed again with saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain a crude product. The crude product was dissolved in acetone, fed with hydrogen chloride gas, stirred for 1 h, and then filtered with suction to obtain the hydrochloride of formula II. The hydrochloride salt of formula II was dissolved in water, and was extracted with aqueous sodium bicarbonate until no gas was generated, dried, concentrated and used (20.5 g, 83.4%).
Embodiment 3
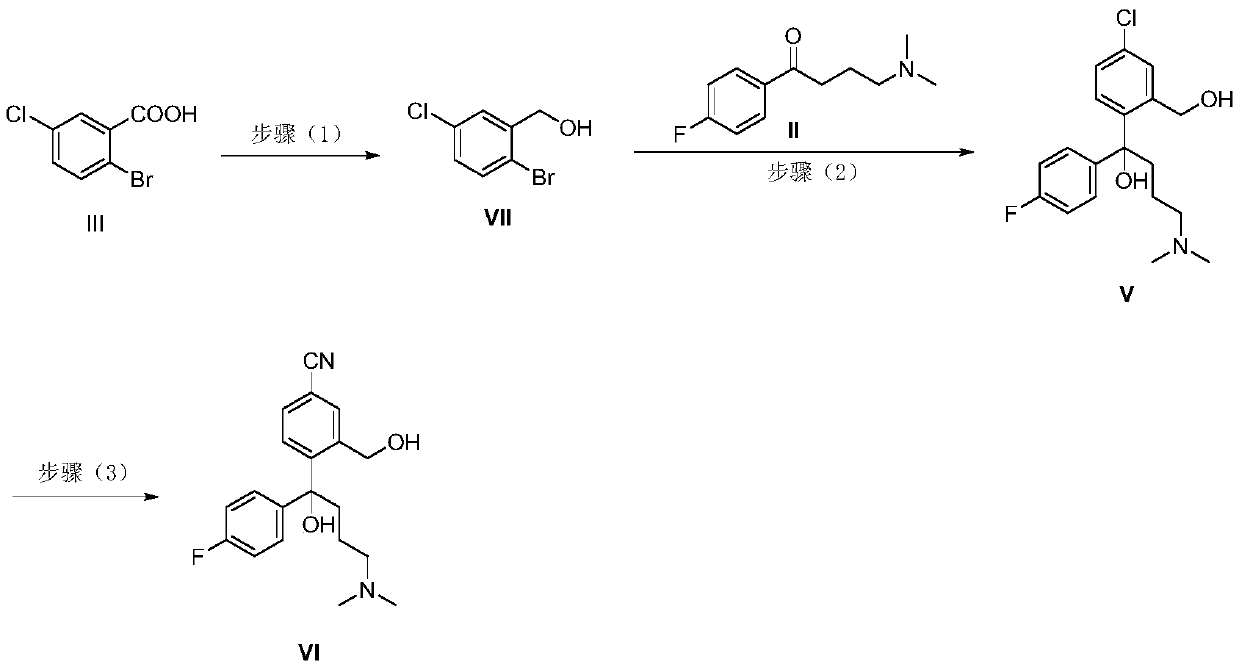
[0045] Example 3 Synthesis of 5-chloro-2-(4-(dimethylamino)-1-(4-fluorophenyl)-1-hydroxybutyl)benzoic acid
[0046] -78°C, under nitrogen protection, carefully drop n-butyl lithium (2.5M / hexane, 0.11mol) into 5-chloro-2-bromobenzoic acid (11.8g, 0.05mol) in anhydrous THF (100 mL ,44.0mL). After the dropwise incubation for 1 h, a solution of 4-(dimethylamino)-1-(4-fluorophenyl)butan-1-one (10.5 g, 0.05 mol) in THF (20 mL) was added dropwise. After the addition, it was raised to -60°C for 2 h, and then saturated ammonium chloride (100 mL) was added dropwise to quench the reaction. The mixture was extracted with ethyl acetate, washed once with saturated ammonium chloride and saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated. The crude product was slurried with dichloromethane to obtain a white solid (15.3 g, 83.5%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com