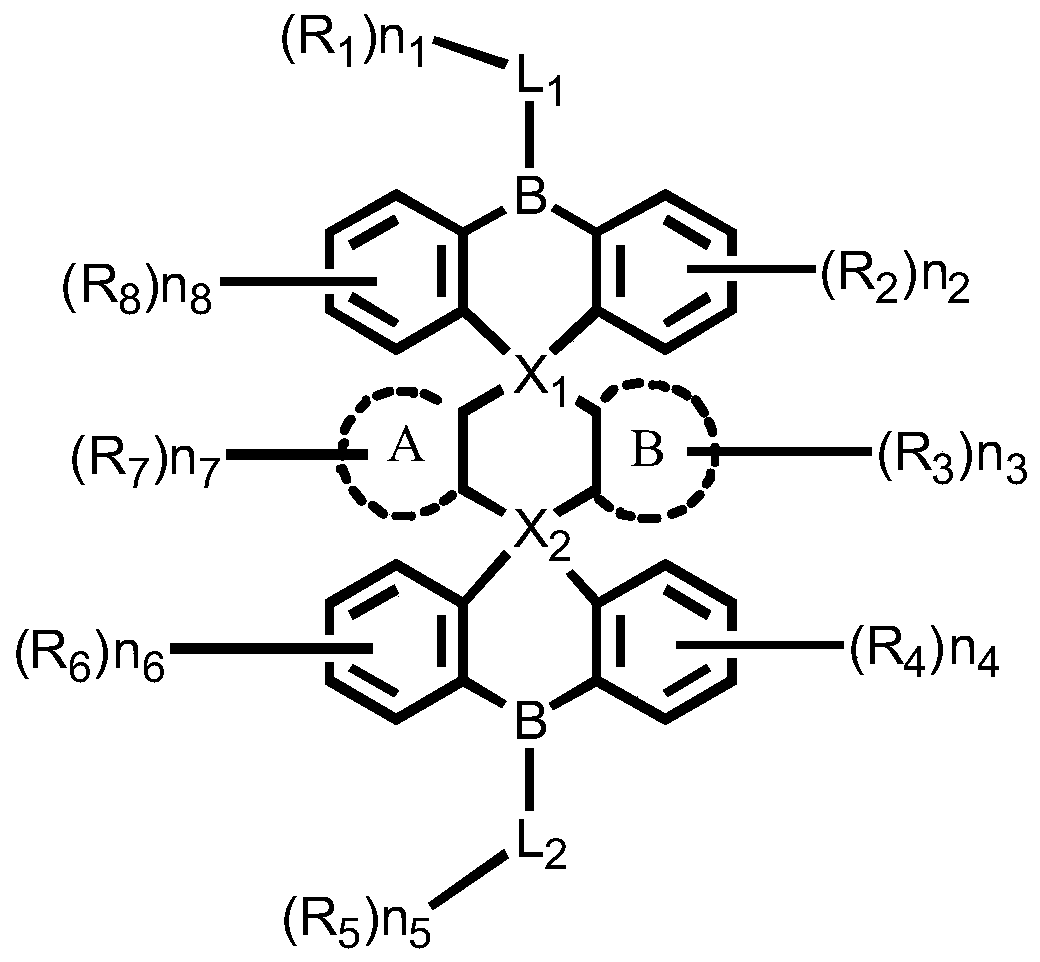
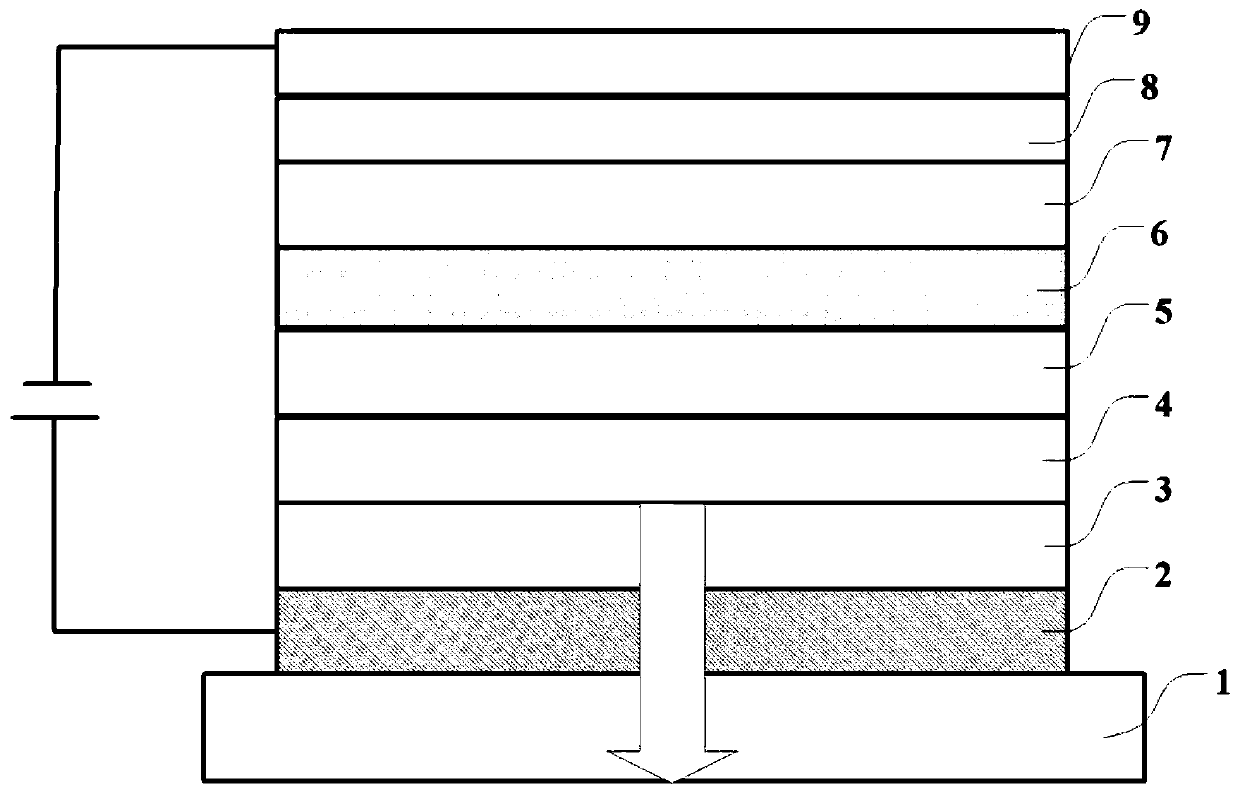
Boron heterocyclic compound, display panel and display device
A technology of compound and boron heterocycle, applied in the field of organic electroluminescent materials, to achieve a high level of dipole orientation rate, improve external quantum efficiency, and reduce the effect of aggregation fluorescence quenching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Synthesis of Compound P001
[0091]
[0092] X02 (4.2 mmol) was weighed and dissolved in THF (30 mL), the resulting solution was cooled to -78°C, kept stirring, and degassed for 20 min. Add nBuLi (4.4mmol, 2.5M n-hexane solution) dropwise to the above solution, and continue stirring for 2h. X01 (2 mmol) was weighed and dissolved in THF (10 mL), and the THF solution of X01 (2 mmol) was added dropwise to the reaction system through a separatory funnel, slowly warmed to room temperature, and stirred overnight. Methanol (1 mL), HCl (2 mL, 1 M) and deionized water (20 mL) were added again to the reaction system dropwise, and the reaction system was continuously stirred. The reaction system was extracted with dichloromethane (3×10mL), the organic phase was separated and collected, the organic phase was washed with saturated sodium chloride solution (30mL), and anhydrous Na 2 SO 4 The organic phase is dried. The solvent was distilled off under reduced pressure to obtain...
Embodiment 2
[0111] Synthesis of Compound P030
[0112]
[0113] X12 (4.3 mmol) was dissolved in 40 mL of anhydrous THF at room temperature under nitrogen atmosphere. NaH (5.8 mmol) was repeatedly washed with n-hexane, and then added to the above solution. After stirring for 1 h, X06 (2.0 mmol) was added and stirred overnight at room temperature. The reaction was quenched by adding methanol and water. Extract with dichloromethane, collect the organic phase, and wash with anhydrous Na 2 SO 4 Dry processing. The dried solution was filtered, and the solvent was removed by a rotary evaporator to obtain a crude product. The crude product was purified by silica gel chromatography using chloroform / n-hexane as the eluent, and finally purified again by sublimation to obtain solid P030 (1.6 mmol, yield 80%).
[0114] MALDI-TOF MS: C 74 h 48 B 2 N 2 o 2 : m / z calculated value: 1018.4; tested value: 1018.6.
[0115] Compound P030 elemental analysis calculated value: C, 87.24; H, 4.75; N...
Embodiment 3
[0117] Synthesis of compound P062
[0118]
[0119] X13 (2.2 mmol) was dissolved in 50 mL of anhydrous THF at room temperature under nitrogen atmosphere. NaH (2.9 mmol) was washed repeatedly with n-hexane, and then slowly added to the above solution. After stirring for 2 h, X10 (1.0 mmol) was added and stirred overnight at room temperature. The reaction was quenched by adding methanol and water. Extract with dichloromethane, collect the organic phase, and wash with anhydrous Na 2 SO 4 Dry processing. The dried solution was filtered, and the solvent was removed by a rotary evaporator to obtain a crude product. The crude product was purified by silica gel chromatography using chloroform / n-hexane as the eluent, and finally purified by sublimation again to obtain solid P062 (0.68 mmol, yield 68%).
[0120] MALDI-TOF MS: C 80 h 60 B 2 N 2 : m / z calculated value: 1070.5; tested value: 1070.7.
[0121] Compound P062 elemental analysis calculated value: C, 89.72; H, 5.65...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com