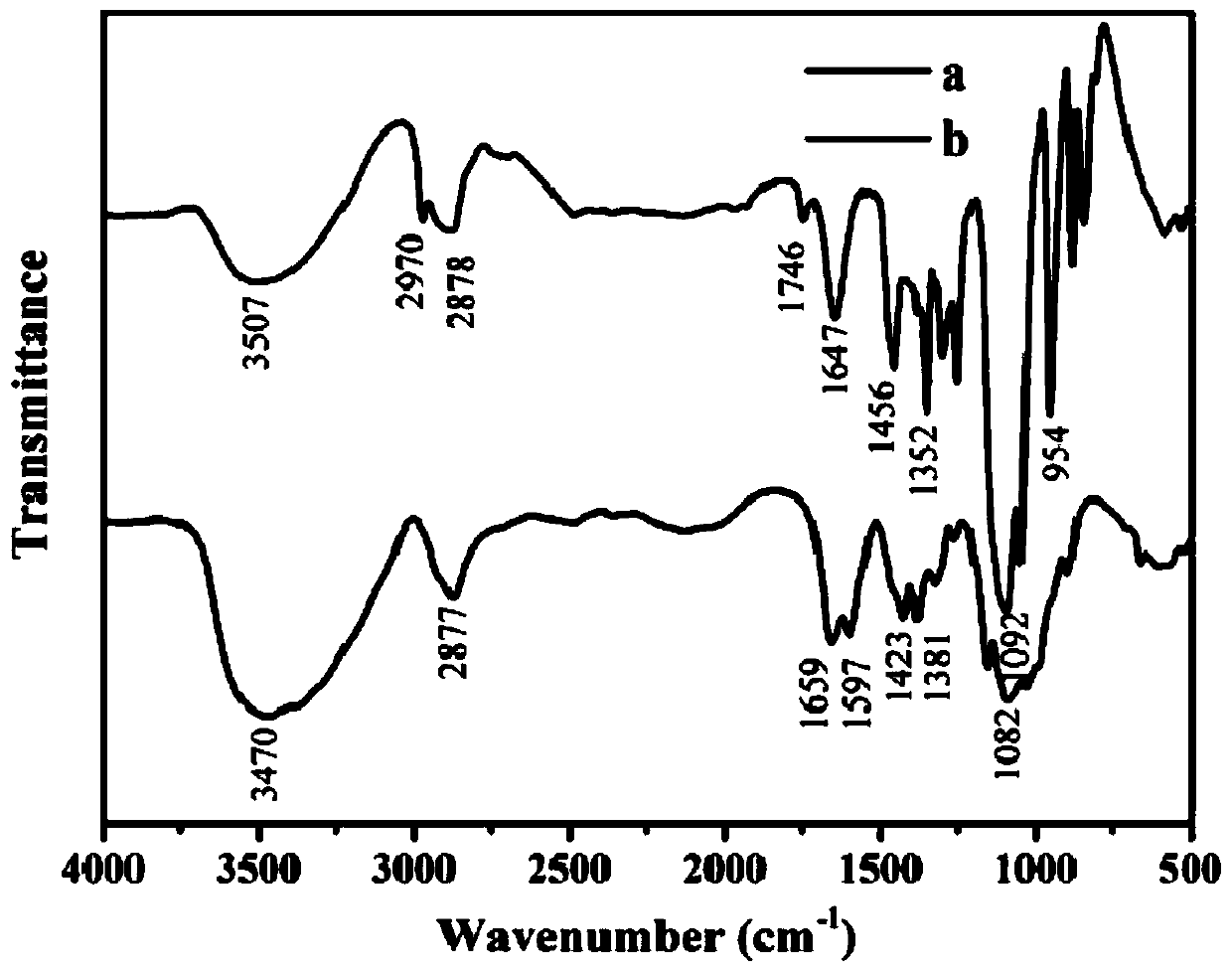
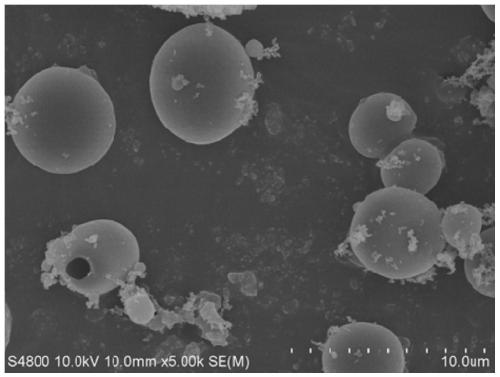
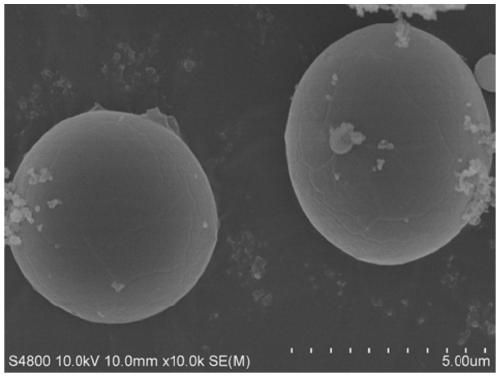
Photo-thermal dual-responsiveness chitosan derivative as well as preparation method and application thereof
A technology of chitosan derivatives and photoresponsiveness, which is applied in the fields of pharmaceutical formulation, drug delivery, and photodisintegration of drugs in vivo, and can solve the problem of microencapsulated emulsions and light sources that are not suitable for embedding bioactive drugs and plant essential oils. The sensitivity needs to be improved, the preparation method is complicated, etc., and the preparation process is easy to scale up, the preparation process is gentle, and the particle size is uniform.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] The preparation of the photothermal sensitive type carboxymethyl chitosan nano-microcapsule loaded with Abamectin in this embodiment comprises:
[0069] (1) Preparation of photothermally sensitive carboxymethyl chitosan derivatives
[0070] ① Take 0.0025mol o-nitrobenzyl alcohol (0.3825g) and 0.0025mol tungalic anhydride (0.94g) respectively and dissolve them in 100mL chloroform solution, add appropriate amount of 4-dimethylaminopyridine (DMAP), under nitrogen protection environment, Reflux reaction at 60°C for 12 hours, part of the chloroform was distilled off under reduced pressure, and then the mixture obtained in the reflux reaction was cooled to room temperature, washed, extracted, and dried to obtain o-nitrobenzyl tungalic anhydride.
[0071] ②At room temperature, mix 0.270g 1-hydroxyl-benzotriazole (HOBt), 1.2g 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC), 2mmol tungalic anhydride ortho Nitrobenzyl ester was dissolved in 50mL of ethanol solution; then the...
Embodiment 2
[0085] The preparation method of the photothermal-sensitive type carboxymethyl chitosan nano-capsule of the load tea tree essential oil of the present embodiment comprises:
[0086] (1) Preparation of photothermally sensitive carboxymethyl chitosan derivatives
[0087] ① Weigh 0.0025mol o-nitrobenzyl alcohol (0.3825g) and 0.0025mol tungalic anhydride (0.94g) and dissolve them in 500mL ethyl acetate solution, add appropriate amount of 4-dimethylaminopyridine (DMAP), nitrogen protection environment Under reflux at 50°C for 24 hours, part of the ethyl acetate was distilled off under reduced pressure, and then the resulting mixture was cooled to room temperature, washed, extracted, and dried to obtain o-nitrobenzyl tungalic anhydride.
[0088]②At room temperature, dissolve 0.270g of 1-hydroxy-benzotriazole (HOBt), 1.2g of dicyclohexylcarbodiimide (DCC), and 2mmol of o-nitrobenzyl tungalic anhydride in 50mL of ethanol solution; then The above solution was added dropwise to 500 mL ...
Embodiment 3
[0098] The preparation method of the photothermal-sensitive type carboxymethyl chitosan nano-microcapsule of load patchouli essential oil of the present embodiment comprises:
[0099] (1) Preparation of photothermally sensitive carboxymethyl chitosan derivatives
[0100] ① Weigh 0.0025mol o-nitrobenzyl alcohol (0.3825g) and 0.0025mol tungalic anhydride (0.94g) and dissolve them in 500mL ethyl acetate solution, add appropriate amount of 4-dimethylaminopyridine (DMAP), nitrogen protection environment Under reflux at 50°C for 24 hours, part of the ethyl acetate was distilled off under reduced pressure, and then the resulting mixture was cooled to room temperature, washed, extracted, and dried to obtain o-nitrobenzyl tungalic anhydride.
[0101] ②At room temperature, add 0.270g of benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU), 1.2g of 1-(3-dimethylaminopropyl)- 3-Ethylcarbodiimide (EDC), 2mmol tungalic anhydride o-nitrobenzyl ester were dissolved in 50mL o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com