Method for converting carbonic acid type salt lake brine into chloride type brine
A salt lake brine and carbonic acid type technology, applied in alkali metal chloride, lithium carbonate;/acid carbonate, lithium halide, etc., can solve the risk of environmental pollution, poor environmental self-purification ability, large acid-base consumption, etc. It can improve the yield of lithium ions, reduce the loss of lithium ions, and improve the efficiency of irradiation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
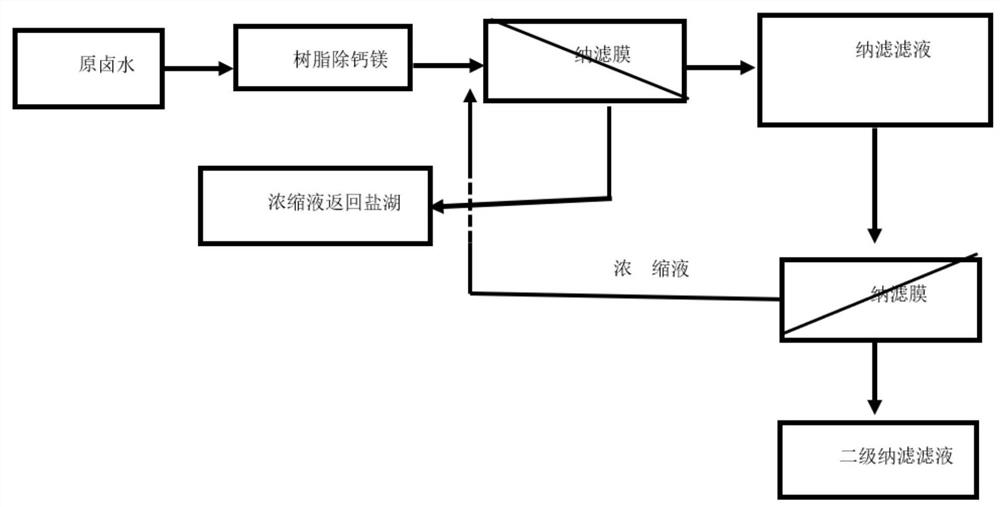
[0045] see figure 1 , according to another preferred embodiment of the present invention, the method for converting the carbonate type salt lake brine into chloride type brine is provided, which is characterized in that it includes the following steps:
[0046] passing the carbonated salt lake brine through an ion exchange resin to remove trace amounts of calcium and magnesium ions in the brine to obtain softened brine;
[0047] Pump the softened brine into the first-stage nanofiltration membrane system, and apply pressure on both sides of the first-stage nanofiltration membrane to form a pressure difference;
[0048] Part of the water, sodium ions, potassium ions, lithium ions, and chloride ions in the softened brine migrate from the high-pressure side to the low-pressure side through the first-stage nanofiltration membrane;
[0049] The high-valent anions on the high-pressure side are enriched to obtain brine rich in high-valent anions, and the brine rich in high-valent anion...
Embodiment 2
[0069] Some carbonate-type salt lakes are rich in lithium ions and carbonate ions, and have high resource endowment. Lithium carbonate can be formed after concentration in salt fields. However, salt lakes also contain a large amount of sulfate ions, polyborate ions, bicarbonate ions, chloride ions, sodium ions, and potassium ions. The existence of a large number of borate ions leads to low radiation efficiency in salt fields. At the same time, a large amount of salts such as mirabilite, sodium carbonate decahydrate, sodium chloride, halite, potassium halite, potassium chloride, and sodium tetraborate are formed during the concentration process of salt fields. A large amount of entrainment loss of lithium ions is caused, resulting in a low yield of lithium ions. At the same time, the phase diagram of the multi-component system is complex, and valuable elements such as potassium ions cannot be separated, resulting in a great waste of resources.
[0070] Therefore, the invention ...
Embodiment 3
[0079] A specific example is further described below with reference to Embodiment 1-2. A kind of lithium-containing carbonate type salt lake brine whose index is Li + : 1g / L, Na + : 90g / L, K + 20g / L, Mg 2+ : 10ppm, Ca 2+ : 0.04ppm, B 2 o 3 : 13g / L; SO 4 2- : 20g / L, CO 3 2- : 20g / L, HCO 3 -: 0.4g / L, chloride ion 120g / L;
[0080] The specific implementation steps are as follows:
[0081] a) The brine is passed through the sodium-type semi-EDTA type chelating resin, and after the ion exchange of the chelating resin, soften the Mg in the brine 2+ ≤0.01ppm, Ca 2+ ≤0.01ppm, after the resin is saturated, use hydrochloric acid or sulfuric acid to regenerate the hydrogen form, and then use sodium hydroxide to regenerate the sodium form;
[0082] b) The softened brine is pumped into the primary nanofiltration system, and the operating pressure of the nanofiltration system is 90kg / cm 2 At the same time, pure water is added to the nanofiltration membrane concentrated solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com