A kind of nanometer material of zinc oxide coating calcium carbonate and preparation method thereof
A technology of nanomaterials and core-shell nanomaterials, which is applied in the field of new inorganic non-metallic materials, can solve the problems of inability to meet the needs of zinc oxide activity, environmental pollution, large particle size, etc., and achieve good coating effect and good coating effect , the effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Disperse 50g of calcium carbonate powder with a particle size of 140nm in 50mL of water and stir in a 100mL round bottom flask, dissolve 1.0mol of zinc nitrate and 3.0mol of urea in the above solution respectively, and react at 100°C , the reaction time is 12h, and CaCO can be obtained after the reaction 3 @Zn(OH) 2 Suspensions of nanomaterials;
[0033] (2) Let the suspension obtained in step (1) stand for 2 hours, vacuum filter it with a Buchner funnel, wash it repeatedly with clean water 3 times, and use 500 mL of clean water each time, and dry the obtained nanomaterials in an oven at 100 ° C for 5 hours , to obtain dry CaCO 3 @Zn(OH) 2 ;
[0034] (3) Then the dried CaCO 3 @Zn(OH) 2 The precursor powder was transferred to a muffle furnace for calcination at 350°C, the heating rate was 2°C / min, and the temperature was kept for 2h to obtain zinc oxide-coated calcium carbonate nanomaterials.
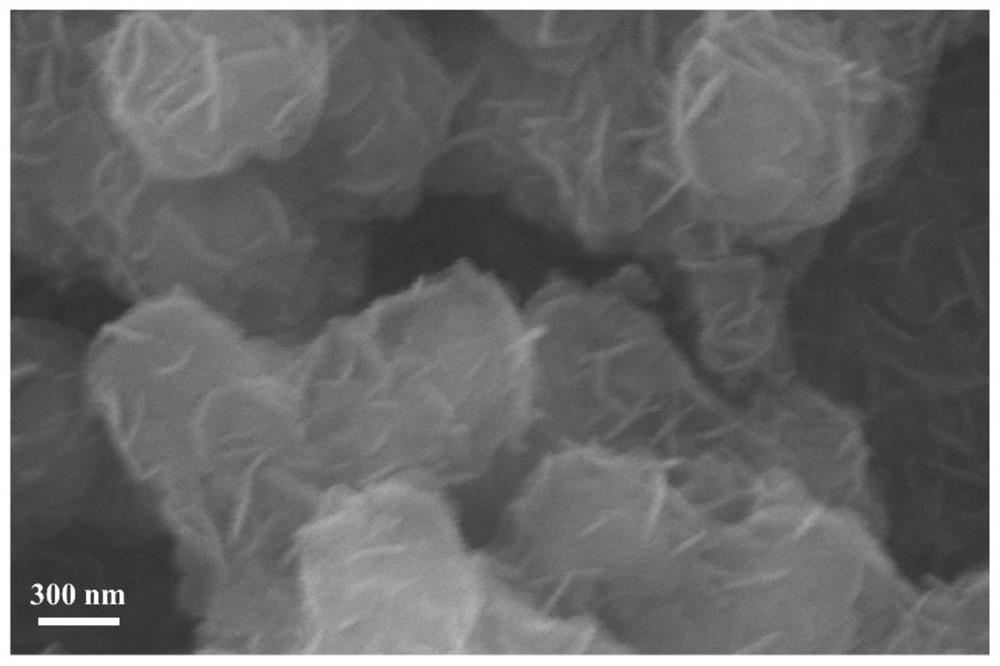
[0035] figure 1 Is the CaCO prepared in this example 3 The scann...
Embodiment 2
[0037] (1) Disperse 50g of calcium carbonate powder with a particle size of 200nm in 50mL of water and stir in a 100mL round bottom flask, dissolve 1.5mol of zinc nitrate and 4.0mol of urea in the above solution, and react at 120°C , the reaction time is 20h, and CaCO can be obtained after the reaction 3 @Zn(OH) 2 Suspensions of nanomaterials;
[0038] (2) Let the suspension obtained in step (1) stand for 2 hours, vacuum filter it with a Buchner funnel, wash it repeatedly with clean water 3 times, and use 500 mL of clean water each time, and dry the obtained nanomaterials in an oven at 100 ° C for 5 hours , to obtain dry CaCO 3 @Zn(OH) 2 ;
[0039] (3) Then the dried CaCO 3 @Zn(OH) 2The precursor powder was transferred to a muffle furnace for calcination at 300°C, the heating rate was 5°C / min, and the temperature was kept for 3h to obtain zinc oxide-coated calcium carbonate nanomaterials.
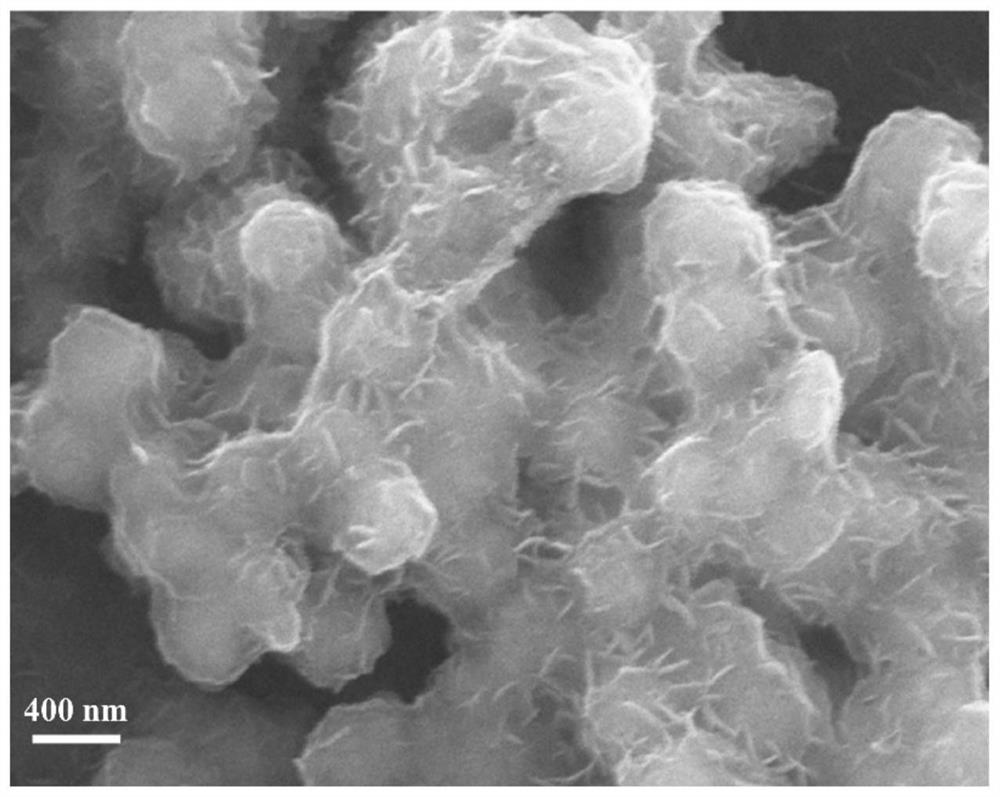
[0040] figure 2 Is the CaCO prepared in this example 3 The scanning electron ...
Embodiment 3
[0042] (1) Disperse 50g of calcium carbonate powder with a particle size of 80nm in 50mL of water and stir in a 100mL round bottom flask, dissolve 0.5mol of zinc nitrate and 2.0mol of urea in the above solution, and react at 90°C , the reaction time is 24h, and CaCO can be obtained after the reaction 3 @Zn(OH) 2 Suspensions of nanomaterials;
[0043] (2) Let the suspension obtained in step (1) stand for 2 hours, vacuum filter it with a Buchner funnel, wash it repeatedly with clean water 3 times, and use 500 mL of clean water each time, and dry the obtained nanomaterials in an oven at 100 ° C for 5 hours , to obtain dry CaCO 3 @Zn(OH) 2 ;
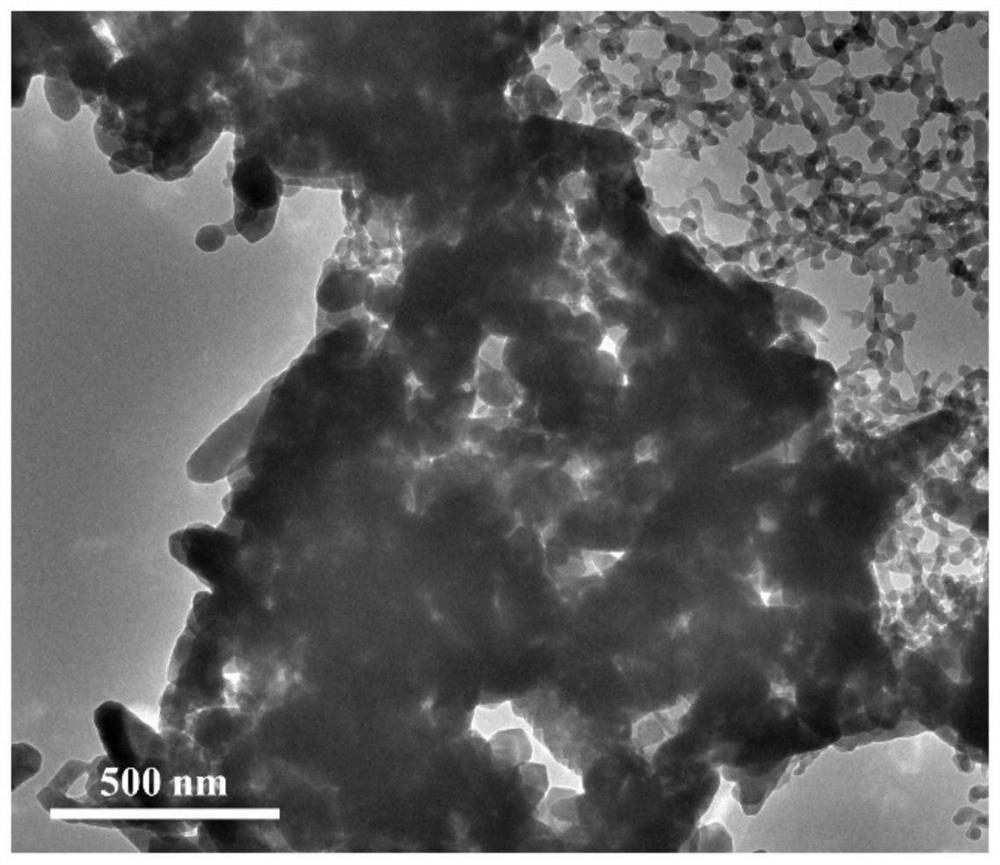
[0044] (3) Then the dried CaCO 3 @Zn(OH) 2 The precursor powder was transferred to a muffle furnace for calcination at 300°C, the heating rate was 1°C / min, and the temperature was kept for 2 hours to obtain nanomaterials coated with zinc oxide calcium carbonate. Zinc oxide can completely coat calcium carbonate, but the particle agglome...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com