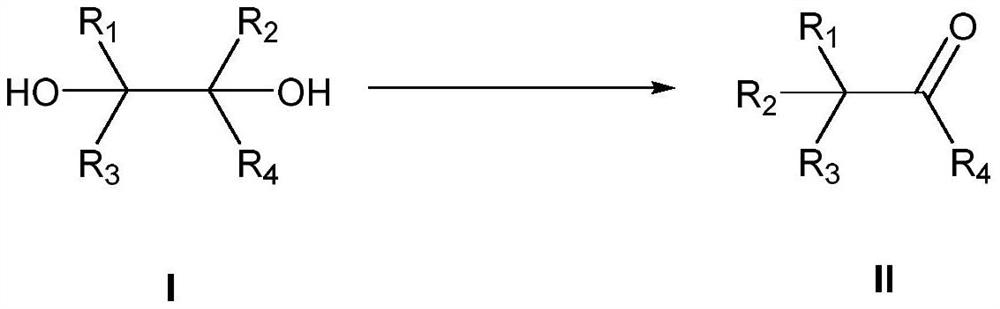
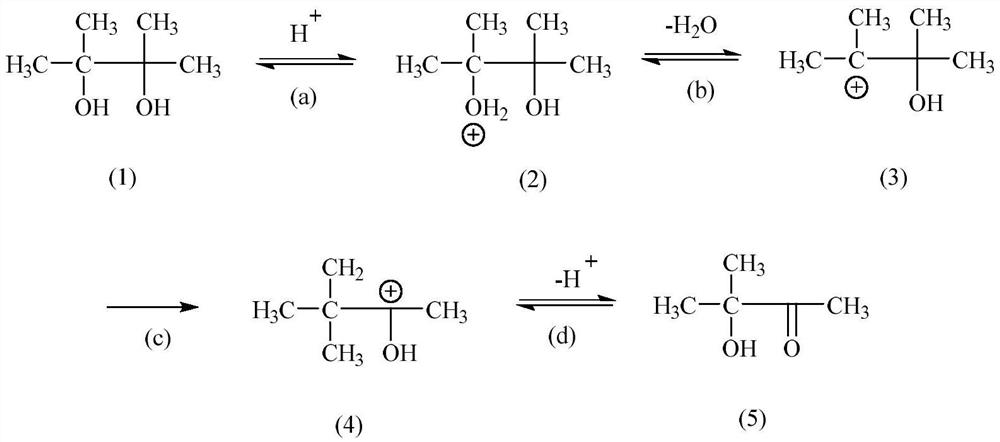
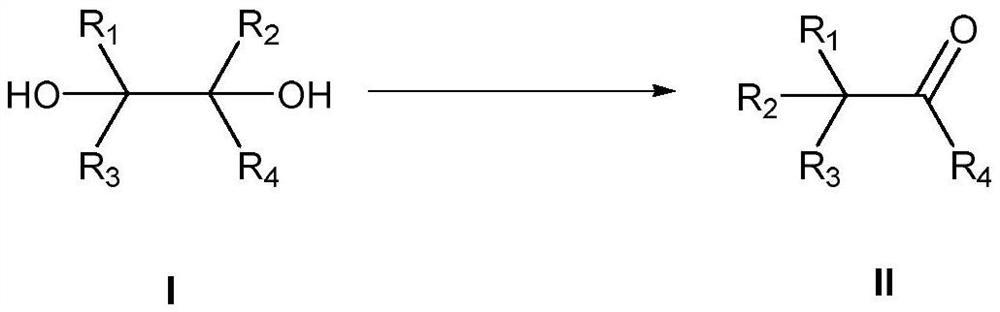
Method for preparing substituted carbonyl compound by catalyzing pinacol rearrangement reaction through molecular sieve
A molecular sieve catalytic frequency, carbonyl compound technology, applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of organic compounds, etc. Wide range of applications, cheap catalysts, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Weigh 50 mg of benzoin hydrochloride, measure 5 mL of toluene with a graduated cylinder, mix and dissolve, slowly add 10 mg of USY-6 molecular sieve, and heat, stir and reflux, and carry out the pinacol rearrangement reaction of the following reaction equation at 110°C :
[0017]
[0018] After reacting for 5 hours, add 10ml of ethyl acetate to dissolve the organic matter, then filter out the molecular sieve, remove a small amount of water from the ethyl acetate solution with anhydrous sodium sulfate, filter, spin dry, and separate by column chromatography to obtain 44.3 mg of the product as di Phenylacetaldehyde, its yield is 88.6%.
[0019] The above-mentioned product is detected as the target compound by nuclear magnetic spectroscopy, and its test data are as follows:
[0020] 1 H NMR (400MHz, CDCl 3 )δ=9.99(d,J=3.4Hz,1H),7.45-7.31(m,6H),7.29-7.22(m,4H)4.93(d,J=3.4Hz,1H)ppm; 13 C NMR (100MHz, CDCl 3 ) δ = 199.9, 135.3, 130.0, 129.9, 127.8, 64.0 ppm.
Embodiment 2
[0022] Weigh 50mg of benzopinacol, measure 5mL of toluene with a measuring cylinder, mix and dissolve, slowly add 10mg of MCM-41 molecular sieve, and heat, stir and reflux, and carry out the pinacol rearrangement reaction of the following reaction equation at 110°C :
[0023]
[0024] After reacting for 3 hours, add 10ml ethyl acetate to dissolve the organic matter, then filter out the molecular sieves, remove a small amount of water from the ethyl acetate solution with anhydrous sodium sulfate, filter, spin dry, and separate by column chromatography to obtain 45.8 mg of the product as three Benzyl acetophenone, its yield is 91.6%.
[0025] The above-mentioned product is detected as the target compound by nuclear magnetic spectroscopy, and its test data are as follows:
[0026] 1 HNMR (400MHz, CDCl 3 )δ=7.15-7.21 (m, 2H), 7.21-7.35 (m, 16H), 7.67–7.73 (m, 2H) ppm; 13 C NMR (100MHz, CDCl 3 ) δ = 71.2, 126.8, 127.7, 127.9, 131.0, 131.2, 131.8, 137.6, 143.3, 199.0 ppm.
Embodiment 3
[0028] Weigh 50mg of 1,2-di-p-tolylmethyl 1,2-diol, measure 5mL of toluene with a graduated cylinder, mix and dissolve, then slowly add 10mg of ZSM-35 molecular sieve, and heat, stir and reflux at 110°C. The pinacol rearrangement reaction of said reaction equation:
[0029]
[0030] After reacting for 4 hours, add 10ml of ethyl acetate to dissolve the organic matter, then filter out the molecular sieve, remove a small amount of water from the ethyl acetate solution with anhydrous sodium sulfate, filter, spin dry, and separate by column chromatography to obtain 45.4 mg of the product as 2 , 2-two p-tolyl acetaldehyde, the yield was 90.8%.
[0031] The above-mentioned product is detected as the target compound by nuclear magnetic spectroscopy, and its test data are as follows:
[0032] 1 H NMR (400MHz, CDCl 3 )δ=9.91(d, 1H, J=2.5Hz), 7.17(m, 4H), 7.10(m, 4H), 4.82(d, J=2.5Hz 1H,), 2.34(s, 6H)ppm; 13 C NMR (100MHz, CDCl 3 ) δ = 198.9, 137.3, 133.4, 129.6, 129.0, 63.4, 21....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com