Preparation method of cefixime delta 3 isomer impurity
A technology of cefixime and isomers, which is applied in the field of preparation of cefixime △3 isomer impurities, achieves the effects of low requirements for reaction equipment, mild reaction conditions, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
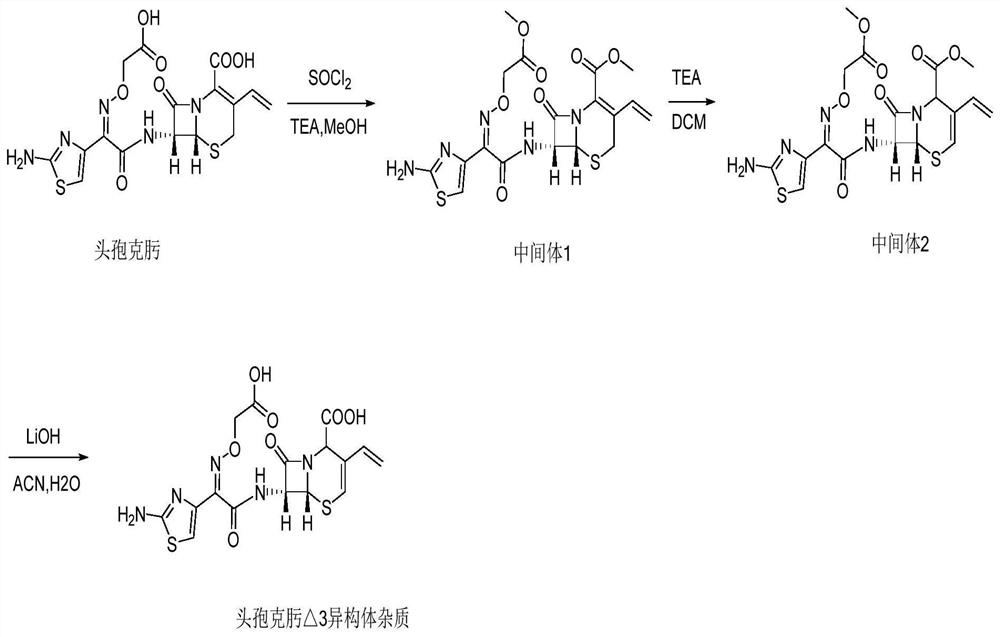
[0044] As attached figure 1 The reaction scheme shown in the reaction scheme for the preparation of cefixime △3 isomer impurities includes the following specific steps:
[0045] a: (6R, 7R)-methyl 7-((Z)-2-(2-aminothiazol-4-yl)-2-((2-2-methoxy-2-oxyethoxy) sub Synthesis of amino)acetamido)-8-oxo-3-vinyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate (Intermediate 1).
[0046] Add cefixime 9.06g (20mmol) and 35mL of anhydrous methanol to a dry 100mL single-necked flask. Add 4.55g (45mmol) of triethylamine slowly with stirring in an ice bath, and then slowly add 5.35g (45mmol) of thionyl chloride. ), after dropping, the temperature is controlled at 65°C and the reaction is refluxed and stirred for 4 hours, and the solvent is removed by rotary evaporation at 40°C. 25mL of dichloromethane is added. After stirring to clear, 50mL of methyl tert-butyl ether is slowly added dropwise to the reaction solution. Become turbid, slowly stir for 20 minutes under temperature control in an ice ...
Embodiment 2
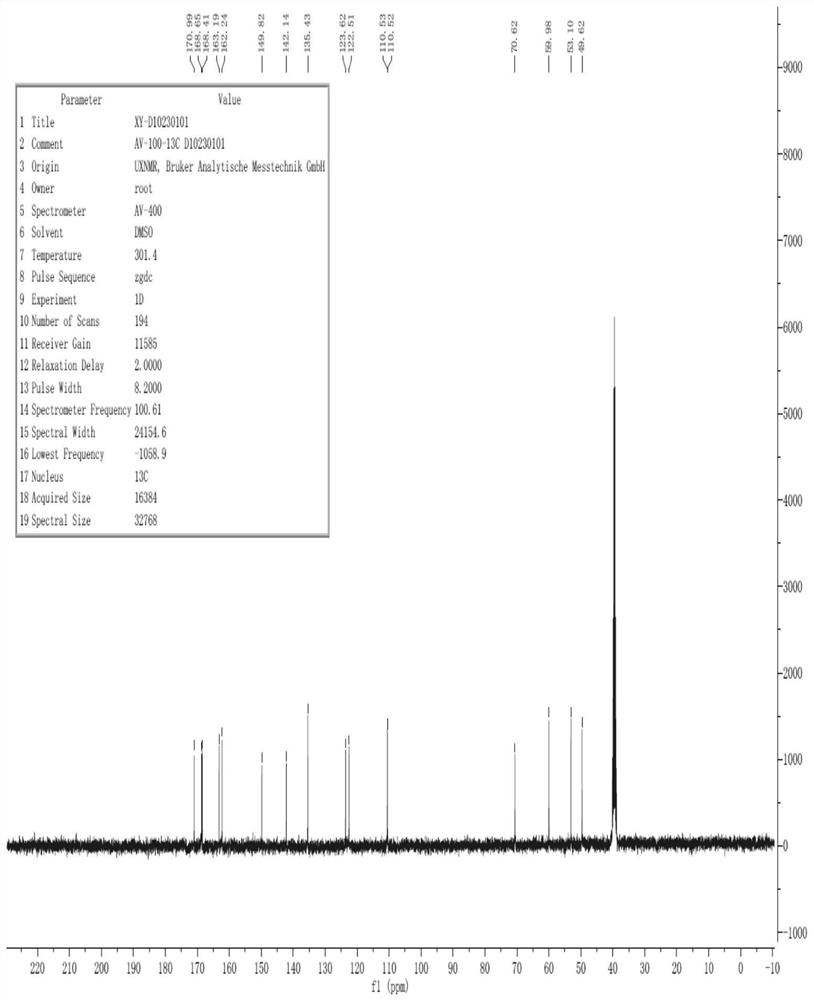
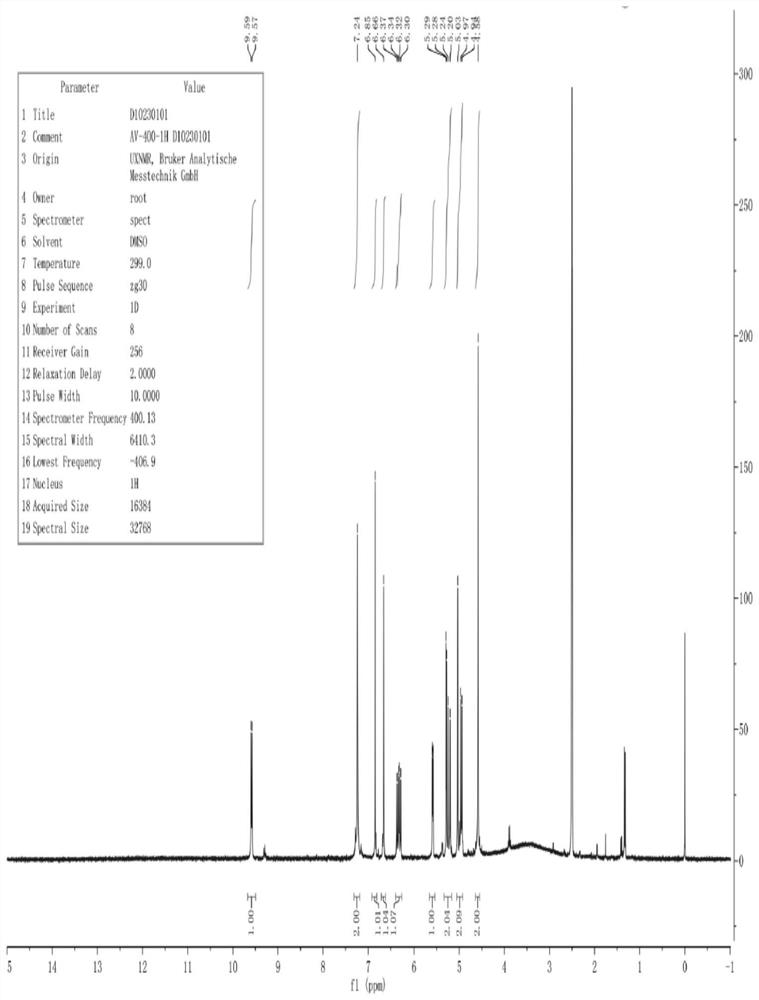
[0052] The hydrogen spectrum and carbon spectrum of the cefixime Δ3 isomer impurities prepared in Example 1 were analyzed to further confirm its structure.
[0053] 1. Hydrogen spectrum analysis
[0054] The number of the impurity hydrogen atom of cefixime △3 isomer is as follows:
[0055]
[0056] The analysis results of the hydrogen spectrum of cefixime △3 isomer impurities are shown in Table 1 below:
[0057] Table 1: Analytical results of hydrogen spectrum of cefixime delta 3 isomer impurities.
[0058] H serial number H type H number chemical shift 1 =CH-16.66 2 =CH-1 6.30~6.37 3=CH 2
[0059] 2. Carbon spectrum analysis
[0060] The number of carbon atoms of cefixime △3 isomer impurity is as follows:
[0061]
[0062] The carbon spectrum analysis results of cefixime △3 isomer impurities are shown in Table 2 below:
[0063] Table 2: Results of carbon spectrum analysis of cefixime delta 3 isomer impurities.
[0064] C serial number Type C chemical shift 1=C-122.51 2 =C123.62 3=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com