Synthesis method of 2, 4, 5-trifluoro-3-methoxybenzoyl chloride
A technology of methoxybenzoyl chloride and methoxybenzoic acid, which is applied in the field of drug synthesis, can solve the problems of high price of tetrafluorophthalic acid, difficulty in refining the target product, long process route, etc., and achieve short time consumption, The effect of reducing the usage amount and speeding up the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
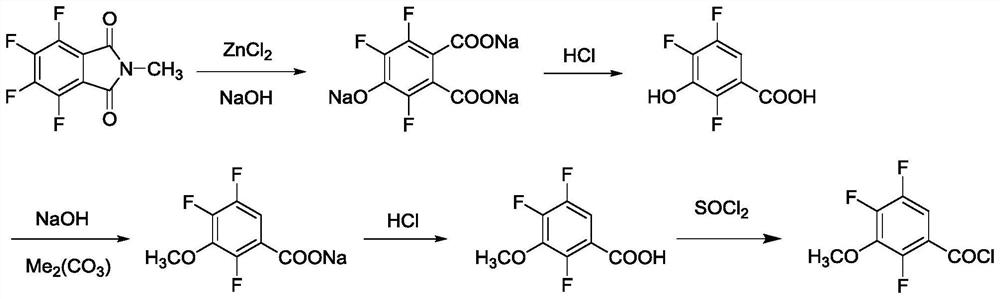
[0054] A kind of synthetic method of present embodiment 2,4,5-trifluoro-3-methoxybenzoyl chloride (process route such as figure 1 shown), including the following steps:
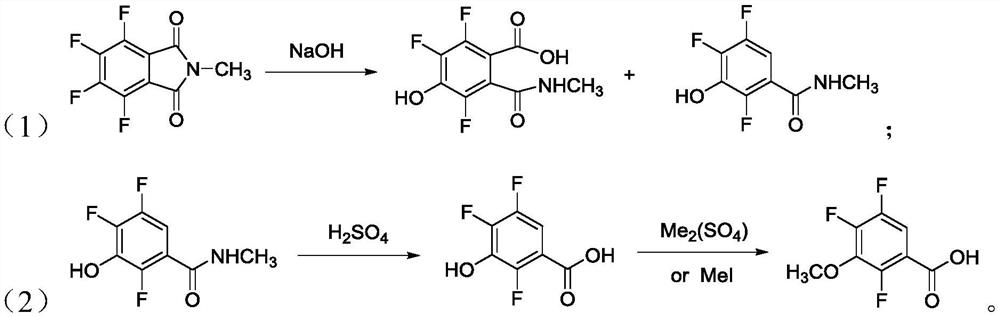
[0055] (1) Using zinc chloride as a catalyst, place N-methyltetrafluorophthalimide in an alkaline environment, and undergo hydrolysis defluorination and hydroxylation to obtain 4-hydroxyl salt-3,5,6 - Trifluorophthalate;
[0056] Specifically, add water 3000kg, N-methyltetrafluorophthalimide 1000kg, 30% liquid caustic soda 2000kg and zinc chloride 50kg (N-methyltetrafluorophthalimide The molar ratio to sodium hydroxide is 1:3.1, and the molar ratio of N-methyltetrafluorophthalimide to zinc chloride is 100:8.5), and the temperature is raised to 100°C for 8 hours, and the reaction ends (liquid The content of N-methyltetrafluorophthalimide in the phase detection reaction system is less than 1%) and then cooled to below 35°C;
[0057] The reaction formula is shown in formula (I):
[0058]
[0059] (2) Add ...
Embodiment 2
[0074] A kind of synthetic method of 2,4,5-trifluoro-3-methoxybenzoyl chloride of the present embodiment comprises the following steps successively:
[0075] (1) Using zinc chloride as a catalyst, place N-methyltetrafluorophthalimide in an alkaline environment, and undergo hydrolysis defluorination and hydroxylation to obtain 4-hydroxyl salt-3,5,6 - Trifluorophthalate;
[0076] Specifically, add 3000kg of water and 1000kg of N-methyltetrafluorophthalimide into the reactor, and then add 30% liquid caustic soda and zinc chloride, wherein, N-methyltetrafluorophthalimide The mol ratio of imine and sodium hydroxide is 1:2, and the mol ratio of N-methyltetrafluorophthalimide and zinc chloride is 100:10; the temperature is raised to 100° C. for 8 hours, and the reaction ends ( The liquid phase detects that the content of N-methyltetrafluorophthalimide in the reaction system is less than 1%) and then cools down to below 35°C;
[0077] (2) Add acid to the reaction system, and obtain ...
Embodiment 3
[0084] A kind of synthetic method of 2,4,5-trifluoro-3-methoxybenzoyl chloride of the present embodiment comprises the following steps successively:
[0085] (1) Using zinc chloride as a catalyst, place N-methyltetrafluorophthalimide in an alkaline environment, and undergo hydrolysis defluorination and hydroxylation to obtain 4-hydroxyl salt-3,5,6 - Trifluorophthalate;
[0086] Specifically, add 3000kg of water and 1000kg of N-methyltetrafluorophthalimide into the reactor, and then add 30% liquid caustic soda and zinc chloride, wherein, N-methyltetrafluorophthalimide The molar ratio of imine to sodium hydroxide is 1:3.5, and the molar ratio of N-methyltetrafluorophthalimide to zinc chloride is 100:7; the temperature is raised to 100° C. for 9 hours, and the reaction ends ( The liquid phase detects that the content of N-methyltetrafluorophthalimide in the reaction system is less than 1%) and then cools down to below 35°C;
[0087] (2) Add acid to the reaction system, and obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com