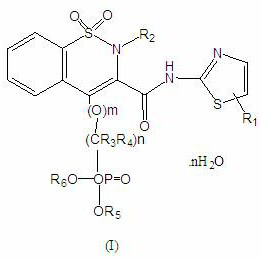
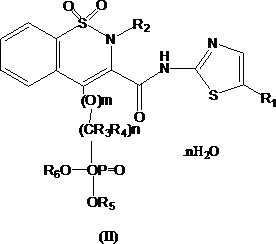
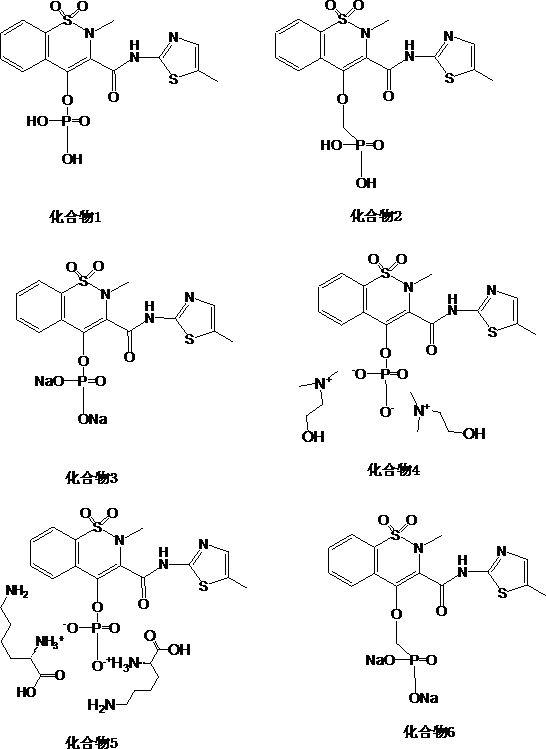
Novel enol non-steroidal compound as well as preparation method and application thereof
An enol, non-steroidal technology, applied in the field of medicine, can solve the problems of unfavorable application, unsafe, blockage and the like of active agents, and achieve the effect of improving oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the preparation of compound 1
[0044]
[0045]
[0046] To a stirred solution of 1 (10 g) in anhydrous DCM (300 ml) was added 2 (21.4 g) dropwise at 0°C. The mixture was then stirred at room temperature for 16 hours, and the reaction was quenched with water. The organic layer was washed with sodium hydroxide solution and water, dried over anhydrous sodium sulfate, and concentrated to dryness. The residue was separated by HPLC to afford about 7.86 g of 3 as a white solid.
[0047] at room temperature and N 2 To a solution of 3 (7.5g) in dry DCM (250ml) was added TMSBr (5.65g). The mixture was then stirred for 6 hours and the solvent was removed under reduced pressure. The residue was separated by HPLC to afford compound 1 (5.45 g, 54.5%) as a white solid.
Embodiment 2
[0048] Embodiment 2: the preparation of compound 2
[0049]
[0050]
[0051] To a stirred solution of 1 (10 g) in anhydrous DCM (300 ml) was added 2 (25.8 g) dropwise at 0°C. The mixture was then stirred at room temperature for 16 hours, and the reaction was quenched with water. The organic layer was washed with sodium hydroxide solution and water, dried over anhydrous sodium sulfate, and concentrated to dryness. The residue was separated by HPLC to give about 6.50 g of 5 as a white solid.
[0052] at room temperature and N 2 To a solution of 3 (6.0 g) in dry DCM (200 ml) was added TFA (20 ml). The mixture was then stirred for 6 hours and the solvent was removed under reduced pressure. The residue was separated by HPLC to afford compound 2 (4.57 g, 45.7%) as a white solid.
Embodiment 3
[0053] Embodiment 3: the preparation of compound 3
[0054]
[0055] Add purified water (20ml) and compound 1 (1.5g) into a 100ml reaction flask and stir, then slowly add sodium hydroxide solution (0.278g sodium hydroxide dissolved in 5ml of water) into the compound 1 solution dropwise to adjust the pH of the solution 9.0 to 10.0, filter, slowly add the filtrate to the stirred isopropanol, stir until a large amount of solids are precipitated, then continue to stir for 1 hour, filter, and dry the filter cake under the condition of 30-40°C to obtain the compound 3 is about 0.85g, and the yield is 56.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com