Natural light induced controlled release drug as well as preparation method and application thereof
A technology of controlled release of drugs and natural light, applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of destroying the balance of blood vessels, increasing the degree of tumor malignancy, and deteriorating the tumor microenvironment, achieving delayed release time and inhibiting tumors. Growth, improve the effect of curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation process of Endo-ICG-ERM of the present invention:
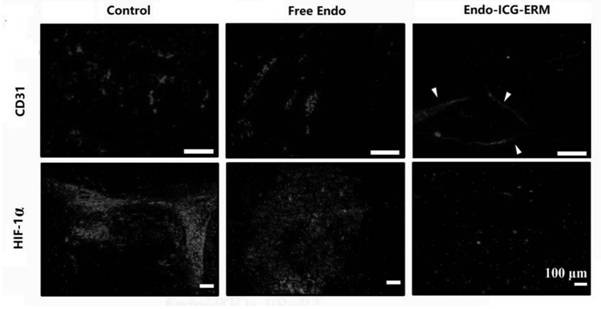
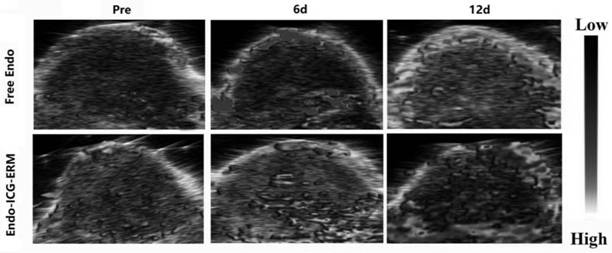
[0043] Such as figure 1 As shown, first extract the red blood cell membrane, take 1mL whole blood, add 10mL hypotonic solution (70mOsm / kg), and lyse at 4°C for 20-30 minutes; centrifuge at 2000rpm for 5min, wash with hypotonic solution 3 times, and wash with isotonic PBS The solution was washed 3 times to obtain red blood cell membrane solution (about 50 μL); then ICG was dissolved in PBS solution, Endo was diluted in PBS, the concentrations of ICG solution and Endo solution were both 10 mg / mL, and the pH of the solution was adjusted to 7.4; followed by ultrasound for drug entrapment on erythrocyte membrane, mix the prepared ICG solution and Endo solution in equal proportions at a volume ratio of 1:1, mix the solution and erythrocyte membrane fluid (mix according to the following ratio: 1mL of whole blood extracted erythrocyte membrane mixed with 1mL ICG solution and 1mL Endo solution, that is, the ini...
Embodiment 2
[0047] The effect of different concentrations of Endo solution on the release of Endo in vivo:
[0048] From Figure 5 It can be seen from the figure that the entrapment capacity of Endo on the red blood cell membrane is related to the initial concentration of Endo, the greater the initial concentration of Endo, the greater the entrapment capacity of Endo on the erythrocyte membrane. When the concentration is increased to 25.0mg / mL, and the entrapment time (ultrasound) exceeds 6 minutes, the entrapment efficiency does not increase significantly.
[0049] From Figure 6It can be seen that the effective release time of Endo also increases with the increase of the initial concentration of Endo, and when the concentration increases to 20.0mg / mL, the release time exceeds 4 days; when the concentration increases to 25.0mg / mL, The release time did not increase significantly.
[0050] Therefore, considering cost-effectiveness, Endo adopts an initial concentration of 20.0mg / mL and a...
Embodiment 3
[0052] The effect of loading different concentrations of ICG solutions on the release of Endo:
[0053] Weigh a certain amount of ICG powder and dissolve it in PBS solution, so that the concentration of ICG solution is gradient to carry out experiments, which are 2mg / mL, 6mg / mL, 10mg / mL, 14mg / mL respectively, and at the same time, use Na 2 HPO 4 and KH 2 PO 4 Adjust the pH to 7.4; dilute Endo with PBS solution to a certain concentration (40.0mg / mL), and adjust the pH to 7.4; mix ICG solution, 1mL of Endo solution and 1mL of ERM solution (red blood cell membrane fluid) extracted from whole blood evenly , sonicated for 6 minutes, and placed at 37° C. for 30 minutes to prepare Endo-ICG-ERM.
[0054] The ICG solution was prepared according to a certain concentration gradient (that is, the initial concentrations of ICG in the above-mentioned entrapment solution were 1 mg / mL, 3 mg / mL, 5 mg / mL, and 7 mg / mL), and the Endo-ICG-ERM drug was prepared, and its exposure to natural light...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap