Cascade catalytic system and method for preparing LLDPE by using system
A technology for catalytic preparation and catalyst, applied in the field of LLDPE, can solve the problems of affecting the mechanical properties of polymers, reducing the molecular weight of polymers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1-1
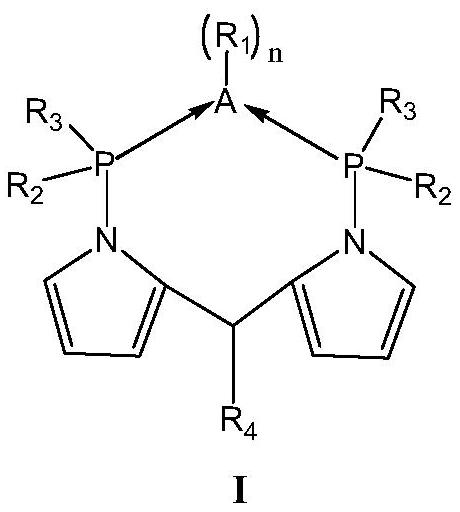
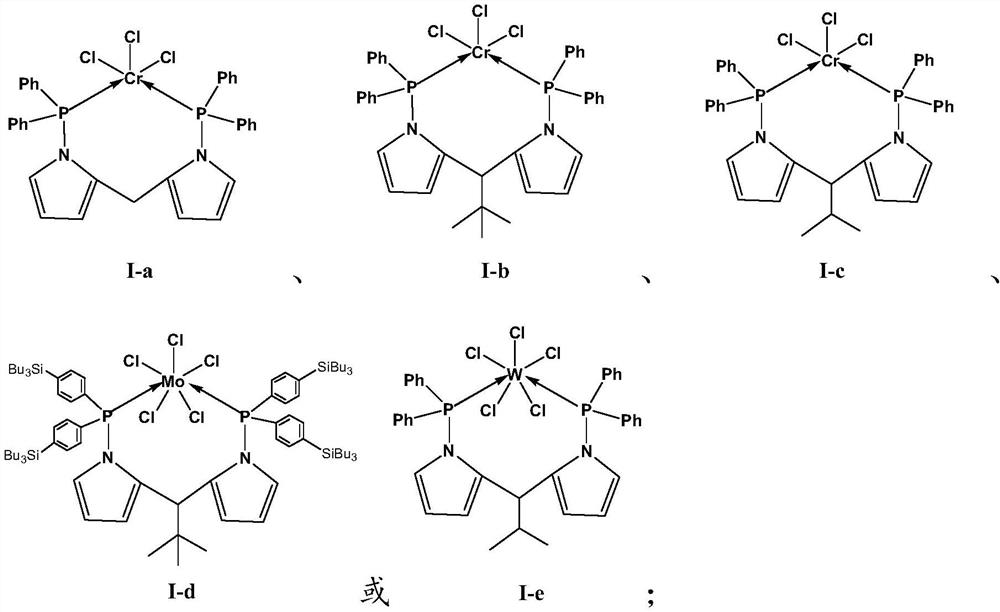
[0134] Dissolve pyrrole (1 mol) and 2-pyrrole formaldehyde (1 mol) in 100 ml of pentane solution, slowly add phosphorus oxychloride (0.2 mol) dropwise at room temperature, react for 1-2 hours, and filter to obtain the insoluble pentane solution Dipyrrole cationic salt, ionic salt dissolved in Ca(OH) 2 In the pentane solution of the suspension, shake vigorously for 5-10 minutes to obtain a pentane solution of dipyrromethene. At -78°C, add excess n-butyllithium (3mol) dropwise to the dipyrromethene pentane solution, slowly warm up to room temperature, stir for 2 to 3 hours, filter and add diphenyl to the solution at -78°C Phosphine chloride (2.5mol), stirred for 2 to 3 hours, overnight at room temperature, purified by column chromatography, and recrystallized to obtain the ligand L 1 . 1 H NMR (400MHz, CDCl 3 ): 3.80~4.0(m, 1H) 7.24~7.35(m, 20H) 7.0~7.10(m, 2H), 5.8~6.1(m, 4H). in the N 2 Add dehydrated methylcyclohexane (200ml), tetrahydrofuran chromium chloride (100μmol) ...
preparation Embodiment 1-2
[0137] At room temperature, trimethylacetaldehyde (2mol) and pyrrole (4.5mol) were dissolved in 100ml of dichloromethane solution, and trifluoroacetic acid (20mL) was dissolved in dichloromethane solution, stirred for 3 to 4 hours to generate a dipyrromethene solution, and at -78°C, add an excess of n-butyllithium (3mol) dropwise to the dipyrromethene solution, slowly warm up to room temperature, stir for 2 to 3 hours, and filter in Diphenylphosphine chloride (2.5mol) was added to the solution at -78°C, stirred for 2-3 hours, left overnight at room temperature, purified by column chromatography, and recrystallized to obtain the ligand L 2 . 1 H NMR (400MHz, CDCl 3 ): 3.68~3.80(m, 1H), 7.24~7.35(m, 20H), 70.0~7.10(m, 2H), 5.8~6.1(m, 4H), 0.95(s, 9H). in the N 2 Add dehydrated toluene (200ml), tetrahydrofuran chromium chloride (100μmol) and ligand L to the replaced Schlenk glass bottle2 (120 μmol), and stirred for 12 minutes to obtain a homogeneous solution of the first catal...
preparation Embodiment 1-3
[0140] The trimethyl acetaldehyde in embodiment 1-2 is changed into isobutyraldehyde, and other conditions are unchanged, obtain ligand L 3 . 1 H NMR (400MHz, CDCl 3 ):3.80~4.0(m, 1H), 7.24~7.35(m, 20H), 7.0~7.10(m, 2H), 5.8~6.1(m, 4H), 0.95(s, 6H), 2.40~2.50(m, 1H ). in the N 2 Add dehydrated Isopar E (200ml), tetrahydrofuran chromium chloride (100μmol) and ligand L to the replaced Schlenk glass bottle 3 (120 μmol), and stirred for 10 minutes to obtain a homogeneous solution of the first catalyst I-c.
[0141]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com