Method for synthesizing 4-methoxy-2-nitroaniline by adopting continuous flow reactor
A technology for methoxyaniline and nitroaniline, applied in the field of organic synthesis, can solve the problems of reduced yield, side reactions, uneven heat exchange, etc., and achieves improved yield and purity, improved yield and purity, and improved reaction safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
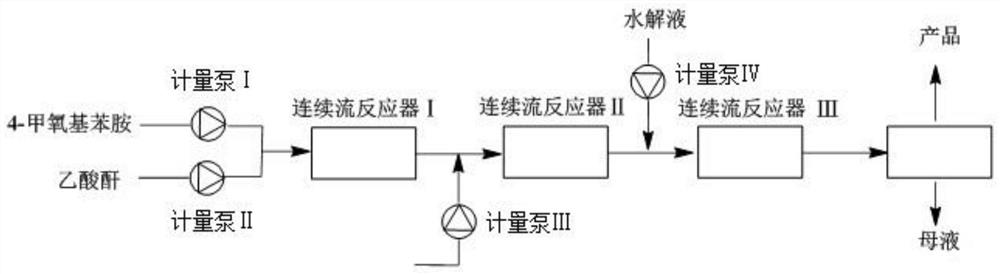
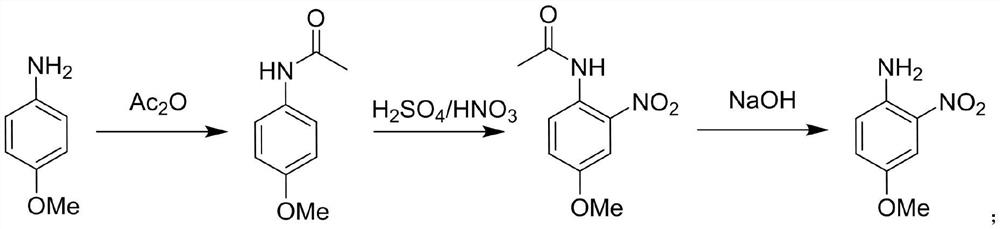
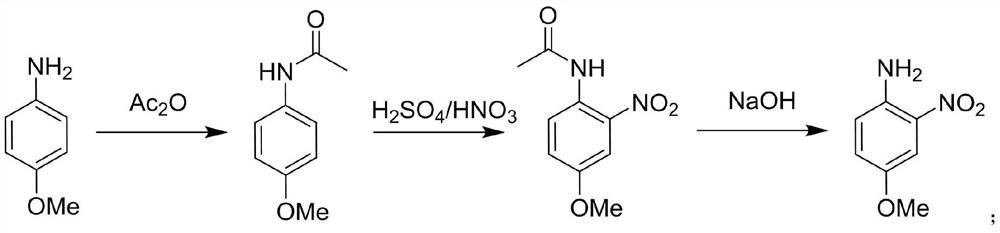
[0039] A method for the synthesis of 4-methoxy-2-nitroaniline using a continuous flow reactor, such as figure 1 Shown, synthetic route is:
[0040]
[0041] Include the following steps:
[0042] (1) Raw material preparation: Pour 96mL of glacial acetic acid and 40g of 4-methoxyaniline, 0.32mol into beaker A and stir until dissolved, weigh 64g of acetic anhydride, pour 0.63mol into beaker B, and mix in beaker C Acid (mass ratio of concentrated sulfuric acid to concentrated nitric acid is 1:1.15) 85g, 0.47mol; prepare 162.6g of 40% NaOH aqueous solution in beaker D, 1.63mol;
[0043] (2) Acetylation reaction: connect beaker A to metering pump Ⅰ, beaker B to metering pump Ⅱ, start metering pump Ⅰ and metering pump Ⅱ at the same time, pour the two solutions into continuous flow reactor Ⅰ, and react at 25°C for 133min , to obtain the reaction solution I containing 4-methoxyacetanilide; wherein, the flow rate of the metering pump I is 5mL / min, and the flow rate of the metering ...
Embodiment 2
[0048] A method for the synthesis of 4-methoxy-2-nitroaniline using a continuous flow reactor, such as figure 1 Shown, synthetic route is:
[0049]
[0050] Include the following steps:
[0051] (1) Raw material preparation: Pour 96mL of glacial acetic acid and 40g of 4-methoxyaniline, 0.32mol into beaker A and stir until dissolved, weigh 64g of acetic anhydride, pour 0.63mol into beaker B, and mix in beaker C Acid (mass ratio of concentrated sulfuric acid to concentrated nitric acid is 1:1.15) 85g, 0.47mol; prepare 162.6g of 40% NaOH aqueous solution in beaker D, 1.63mol;
[0052] (2) Acetylation reaction: connect beaker A to metering pump Ⅰ, beaker B to metering pump Ⅱ, start metering pump Ⅰ and metering pump Ⅱ at the same time, pour the two solutions into continuous flow reactor Ⅰ, and react at 25°C for 133min , to obtain the reaction solution I containing 4-methoxyacetanilide; wherein, the flow rate of the metering pump I is 5mL / min, and the flow rate of the metering ...
Embodiment 3
[0057] A method for the synthesis of 4-methoxy-2-nitroaniline using a continuous flow reactor, such as figure 1 Shown, synthetic route is:
[0058]
[0059] Include the following steps:
[0060] (1) Raw material preparation: Pour 96mL of glacial acetic acid and 40g of 4-methoxyaniline, 0.32mol into beaker A and stir until dissolved, weigh 64g of acetic anhydride, pour 0.63mol into beaker B, and mix in beaker C Acid (mass ratio of concentrated sulfuric acid to concentrated nitric acid is 1:1.15) 85g, 0.47mol; prepare 162.6g of 40% NaOH aqueous solution in beaker D, 1.63mol;
[0061] (2) Acetylation reaction: connect beaker A to metering pump Ⅰ, beaker B to metering pump Ⅱ, start metering pump Ⅰ and metering pump Ⅱ at the same time, pour the two solutions into continuous flow reactor Ⅰ, and react at 25°C for 13.3 min, to obtain the reaction solution I containing 4-methoxyacetanilide; wherein, the flow rate of the metering pump I is 50mL / min, and the flow rate of the meterin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com