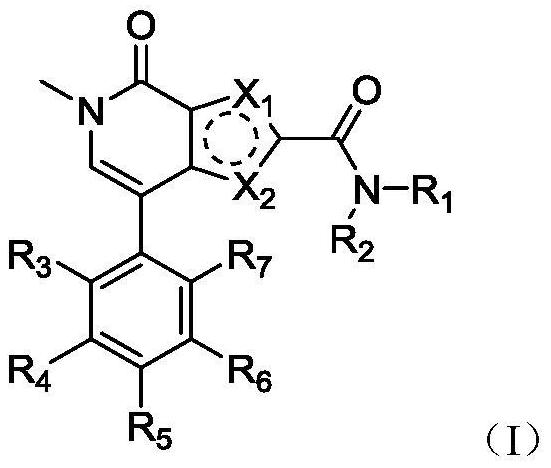
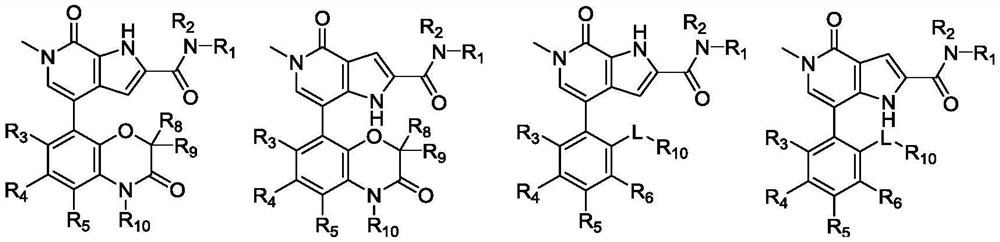
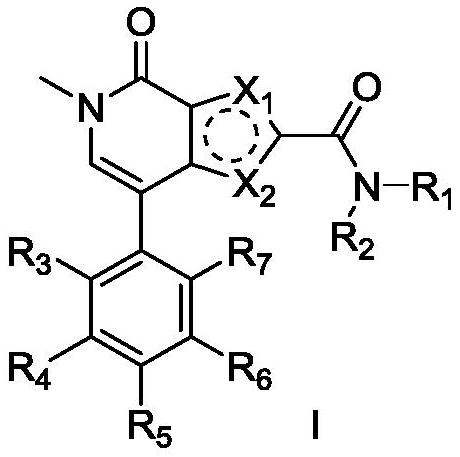
A class of pyrrole amidopyridone compounds, preparation method and use
一种吡咯酰胺、化合物的技术,应用在药物化学领域,能够解决选择性不够突出、BRD蛋白抑制剂作用效果尚待提高、小分子抑制剂结构类型少等问题,达到抑制多种肿瘤细胞的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0319] Example 1: N-ethyl-7-(2-(4-fluoro-2,6-dimethylphenoxy)-5-(2-hydroxypropyl-2-yl)phenyl)-5- Methyl-4-oxo-4,5-dihydro-1H-pyrrolo[3,2-c]pyridine-2-amide
[0320]
[0321] Step 1: Dissolve Intermediate A (100mg, 0.34mmol) in ethanol (10mL), add water (5mL) and sodium hydroxide (53mg, 1.32mmol), and heat to 80°C for 4h. The reaction solution was concentrated to remove most of the ethanol, a small amount of water was added, the pH value was adjusted to 3-4 with dilute hydrochloric acid, and lyophilized to obtain 7-bromo-5-methyl-4-oxo-4,5-dihydro-1H-pyrrole[ 3,2-c]pyridine-2-carboxylic acid (90 mg, white solid). 1 H NMR (400MHz, DMSO_d6): δ 13.13 (brs, 1H), 12.39 (s, 1H), 7.87 (s, 1H), 7.20 (s, 1H), 3.46 (s, 3H).
[0322] The second step: 7-bromo-5-methyl-4-oxo-4,5-dihydro-1H-pyrrole[3,2-c]pyridine-2-carboxylic acid (90mg, 0.33mmol) was dissolved in In DMF (5mL), ethylamine hydrochloride (54mg, 0.67mmol) and N,N-isopropylethyldiamine DIEA (213mg, 1.65mmol) were added suc...
Embodiment 2
[0324] Example 2: N-ethyl-5-methyl-4-oxo-7-(2,2,4-trimethyl-6-(methylsulfone)-3-oxo-3,4-dihydro -2H-Benzo[b][1,4]oxazin-8-yl)-4,5-dihydro-1H-pyrrole[3,2-c]pyridine-2-amide
[0325]
[0326] Under nitrogen protection, 8-bromo-2,2,4-trimethyl-6-(methylsulfonyl)-2H-benzo[b][1,4]oxazin-3(4H)-one (35mg, 0.1mmol) was dissolved in DMF (3mL), and bis-pinacol boroester (51mg, 0.2mmol), cesium carbonate (98mg, 0.3mmol) and Pd(dppf)Cl 2 (7mg, 0.01mmol), heated to 110 degrees and stirred for 2 hours, then added 7-bromo-N-ethyl-5-methyl-4-oxo-4,5-dihydro-1H-pyrrole[3,2 -c] Pyridine-2-amide (30 mg, 0.1 mmol), continue stirring overnight. Cool down to room temperature, add water, extract with dichloromethane DCM, dry the organic phase with anhydrous sodium sulfate, concentrate, and purify by preparative chromatography to obtain N-ethyl-5-methyl-4-oxo-7-(2,2,4- Trimethyl-6-(methylsulfone)-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazin-8-yl)-4,5-dihydro - 1H-pyrrole[2,3-c]pyridine-2-amide (3....
Embodiment 3
[0327] Example 3: N-(5-(2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindoline-4-yl)pent-4-yn-1-yl) -7-(2-(4-fluoro-2,6-dimethylphenoxy)-5-(2-hydroxypropyl-2-yl)phenyl)-5-methyl-4-oxo- 4,5-Dihydro-1H-pyrrole[3,2-c]pyridine-2-amide
[0328]
[0329] The first step: Intermediate A (500mg, 1.68mmol) and 2-(4-(4-fluoro-2,6-dimethylphenoxy)-3-(4,4,5,5-tetramethyl 1,3,2-dioxaborolan-2-yl)phenyl)propan-2-ol was dissolved in DMF (10 mL), cesium carbonate Cs was added 2 CO 3 (1.6g, 4.91mmol) and Pd(dppf)Cl 2 (245mg, 0.33mmol), under the protection of nitrogen, the temperature was raised to 100°C to react overnight. Directly concentrated, the residue was purified by column chromatography to obtain 7-(2-(4-fluoro-2,6-dimethylphenoxy)-5-(2-hydroxypropyl-2-yl)phenyl)-5 - Ethyl methyl-4-oxo-4,5-dihydro-1H-pyrrole[3,2-c]pyridine-2-carboxylate (420 mg, white solid). LC-MS: m / z 493.5[M+H] + .
[0330] Second step: Sodium hydroxide NaOH (136 mg, 1.68 mmol) was added to 7-(2-(4-fluoro-2,6-dimet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com