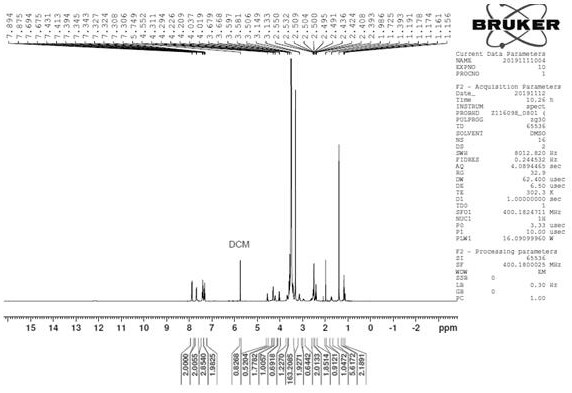
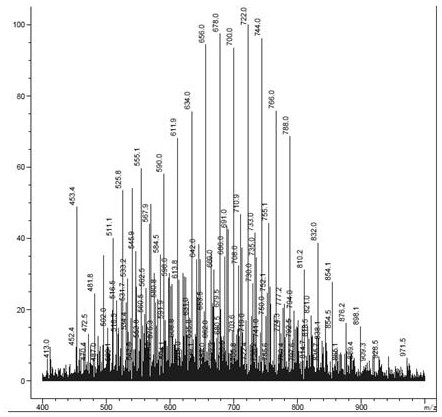
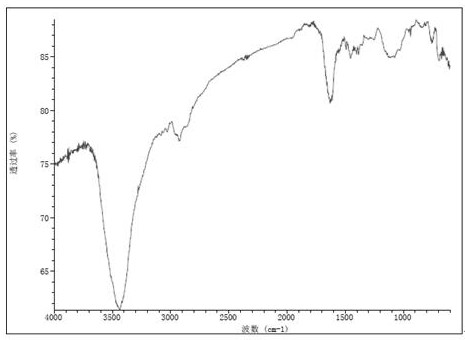
Sulfur-containing polyethylene glycol modified resin
A polyethylene glycol and resin technology, which is applied in the field of sulfur-containing polyethylene glycol modified resins, can solve the problems of substandard performance of modified polyethylene glycol resins and difficult preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1H2
[0173] Example 1H 2 N-PEG (8~12) -CH 2 CH 2 CH 2 -S-CH 2 CH 2 -COOH (compound 7, R 3 =H)
[0174] 1) HO-PEG (8~12) -CH 2 CH 2 CH2 -S-CH 2 CH 2 -COOtBu synthesis
[0175] Dissolve 1200g (about 2.4mol) of APEG500 in 7L of 1,4-dioxane, under nitrogen protection, add 889g (6mol) of tert-butyl 2-mercaptoacetate, raise the temperature to 55°C, add 93.5g of (0.3 mol) AIBN, when the temperature stabilized to 55°C, the nitrogen protection was removed, and the reaction was carried out at 55°C for 13h, and the reaction was complete by MS detection.
[0176] About 4L of 1,4-dioxane was removed by rotary evaporation under reduced pressure, 10L of water was added, and petroleum ether (5L×3) was used to extract a large amount of unreacted tert-butyl 2-mercaptoacetate. The aqueous layer was adjusted to pH=4 with 40% phosphoric acid (a small amount of acid is enough), extracted with dichloromethane (5L*2), washed once with water, dried with anhydrous sodium sulfate, and evaporate...
Embodiment 2
[0186] Example 2Me-HN-PEG (8~12) -CH 2 CH 2 CH 2 -S-CH 2 CH 2 COOH (compound 9, R 3 = Hydrogen, R 5 = methyl)
[0187] 1) Me-NH-PEG (8~12) -CH 2 CH 2 CH 2 -S-CH 2 CH 2 -COOtBu
[0188] 70g (0.1mol) Tos-O-PEG (8~12) -CH 2 CH 2 CH 2 -S-CH 2 CH 2 -COOtBu was dissolved in 400ml of tetrahydrofuran, cooled to -60°C, and 100ml of 3M methylamine tetrahydrofuran solution was added dropwise. After the addition was complete, it was kept at -60°C and stirred for 3 hours. The solvent was evaporated to dryness under reduced pressure to obtain a pale yellow oil, 400ml of water was added to dissolve the oil, the aqueous phase was extracted with ethyl acetate (200ml×3), the acetic acid was washed once with 200ml of saturated brine, dried over anhydrous sodium sulfate, and evaporated under reduced pressure. On drying, 53 g of a yellow oil were obtained.
[0189] 2) Me-NH-PEG (8~12) -CH 2 CH 2 CH 2 -S-CH 2 CH 2 -COOH
[0190] 35g (52mmol) NH 2 -PEG (20~30) -CH 2 CH...
Embodiment 3
[0193] NH2-CH2-CH2-S-CH2CH2CH2-PEG(30~40)-CH2CH2COOH (Compound 16, R1=H, R2=H)
[0194] 1) Synthesis of Boc-NH-CH2-CH2-S-CH2CH2CH2-PEG(30~40)-OH (compound 14)
[0195] Dissolve 150g (about 0.1mol) of APEG1500 in 600ml of 1,4 dioxane, under nitrogen protection, add 106g (0.6mol) of Boc-cysteamine (compound 13), heat up to 55°C, add 3.3g of ( 0.02mol) AIBN, when the temperature stabilized to 55°C, remove the nitrogen protection, react at 55°C for 9h, and MS detected that the reaction was complete.
[0196] Remove about 400ml of 1,4-dioxane by rotary evaporation under reduced pressure, add 400ml of water, and extract a large amount of unreacted Boc-cysteamine with petroleum ether (400ml×3). The aqueous layer was adjusted to pH=4 with 40% phosphoric acid, extracted with dichloromethane (300ml×2), washed once with water, dried with anhydrous sodium sulfate, and evaporated to dryness under reduced pressure to obtain Boc-NH-CH 2 -CH 2 -S-CH 2 CH 2 CH 2 -PEG (30~40) -OH103g.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com