Uric acid test kit capable of eliminating drug interference of calcium dobesilate and etamsylate in serum and preparation method thereof
A technology of calcium dobesilate and ethylamine sulfonate, which is applied in the field of medical testing, can solve problems such as negative deviation, misjudgment of doctor's diagnosis and treatment, consumption of uric acid, etc., to achieve the elimination of negative interference, precise medication and treatment, and accuracy high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
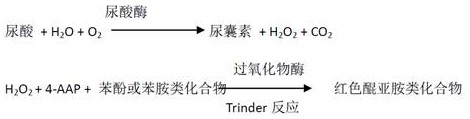
[0063] Reagent R1 components:
[0064] Tris buffer 50mM PH8.0;
[0065] Peroxidase 9 ku / L;
[0066] Ascorbate Oxidase 6 ku / L;
[0067] Vanadium pentoxide 1 mM;
[0068] 4-AAP 3 mM;
[0069] TRITON X-100 0.2%;
[0070] Borax 1%
[0071] Reagent R2 components:
[0072] Tris buffer 50 mM PH8.0
[0073] Uricase 9 ku / L
[0074] Bovine Serum Albumin 0.1%
[0075] TOOS 10 mM
[0076] Borax 1.0%
[0077] TRITON 0.1%.
Embodiment 2
[0079] Reagent R1 components:
[0080] HEPES buffer 50mM PH8.0
[0081] Peroxidase 7.2 ku / L
[0082] Ascorbate Oxidase 6.4 ku / L
[0083] Sodium ferrate 1 mM
[0084] 4-AAP 3 mM
[0085] TRITON X-100 0.2%
[0086] Gentamicin Sulfate 0.1%;
[0087] Reagent R2 components:
[0088] HEPES buffer 50 mM PH8.0
[0089] Uricase 11.2 ku / L
[0090] Bovine Serum Albumin 0.1%
[0091] TOOS 10 mM
[0092] Gentamicin Sulfate 0.1%
[0093] TRITON 0.1%.
Embodiment 3
[0095] Reagent R1 components:
[0096] Phosphate Buffer 50mM PH8.0
[0097] Peroxidase 4.6 ku / L
[0098] Ascorbate Oxidase 10 ku / L
[0099] Sodium metavanadate 2 mM
[0100] 4-AAP 3mM
[0101] TRITON X-100 0.2%
[0103] Reagent R2 components:
[0104] Phosphate Buffer 50mM PH8.0
[0105] Uricase 20 ku / L
[0106] Bovine Serum Albumin 0.1%
[0107] TOOS 10mM
[0108] Sodium azide 0.1%
[0109] TRITON X-100 0.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com