Method for continuously and quickly preparing propiolic acid and derivatives thereof by using microreactor
A kind of microreactor, technology of propynoic acid, applied in the field of synthesis of propynoic acid and its derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A method for continuously and rapidly preparing propiolic acid and derivatives thereof by using a microreactor, the main steps of which are as follows:
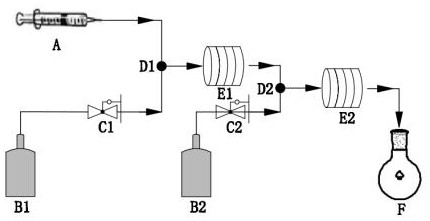
[0027] First, under anhydrous and oxygen-free conditions, add 20.0 g metallic magnesium, 6.0 g elemental iodine and 200 mL anhydrous ether into the reactor and stir evenly. After the elemental iodine is dissolved, slowly add 1.0 mol ethyl bromide dropwise at It is advisable to keep the liquid boiling, and then heat to 60 after the dropwise addition o C was refluxed for 180 min to obtain ethyl magnesium bromide and ether mixed solution; then as figure 1 As shown, the ether solution containing ethylmagnesium bromide and acetylene gas are respectively injected into the micro-mixer D1 (T-type tee, inner diameter: 0.021 mm) by the pump A1 and the flow controller C1 according to the molar ratio of 1:1. After mixing in the medium, enter the microchannel reactor E1 (inner diameter: 0.01mm) at -20 o Under the condition of C,...
Embodiment 2
[0029] A method for continuously and rapidly preparing propiolic acid and derivatives thereof by using a microreactor, the main steps of which are as follows:
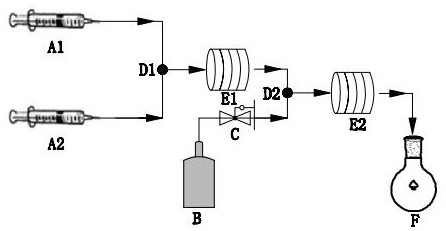
[0030] First, under anhydrous and oxygen-free conditions, add 20.0 g metallic magnesium, 6.0 g elemental iodine and 200 mL anhydrous ether into the reactor and stir evenly. After the elemental iodine dissolves, slowly add 1 mol of vinyl chloride dropwise at a rate of It is advisable to keep the liquid boiling, and then heat to 100 o C was refluxed for 120 min to obtain vinylmagnesium chloride and ether mixed solution; then as figure 2 As shown, the ether solution containing vinylmagnesium chloride and phenylacetylene were respectively injected into the micro-mixer D1 (T-type tee, inner diameter: 1 mm) by the pumps A1 and A2 according to the molar ratio of 1:0.8. Channel reactor E1 (inner diameter: 1 mm), at 40 o Under the condition of C for 10 s, a Grignard exchange reaction occurred to obtain phenylethynylmagnesium...
Embodiment 3
[0032] A method for continuously and rapidly preparing propiolic acid and derivatives thereof by using a microreactor, the main steps of which are as follows:
[0033] First, under anhydrous and oxygen-free conditions, add 20.0 g metallic magnesium, 6.0 g iodine element and 200 mL anhydrous ether into the reactor and stir evenly. After the iodine element dissolves, slowly add 1 mol of bromobenzene dropwise at a rate of It is advisable to keep the liquid boiling, and then heat to 100 o C was refluxed for 60 min to obtain phenylmagnesium bromide and ether mixed solution; then as figure 2As shown, the diethyl ether solution containing phenylmagnesium bromide and 4-methylphenylacetylene were injected into the micro-mixer D1 (T-type tee, inner diameter: 10 mm) by the pumps A1 and A2 according to the molar ratio of 1:1.5. After mixing in the medium, enter the microchannel reactor E1 (inner diameter: 10 mm) at 40 o Under the condition of C, the reaction was carried out for 5 min, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com