Preparation method of high-purity citric acid chelated copper
A technology of citric acid and chelated copper, applied in the preparation of organic compounds, carboxylates, carboxylates, etc., can solve the problems of high reaction temperature, slow reaction rate, easy coating of raw materials, etc., and achieve high purity , The effect of short reaction time and good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] In order to overcome the problems of high reaction temperature, slow reaction rate, product precipitation and easy coating of raw materials, and impure products in the preparation process of citric acid chelated copper, the invention provides a method for preparing high-purity citric acid chelated copper , including the following steps:
[0022] S1: first dissolve citric acid in desalinated water, make a citric acid solution and put it into an enamel reaction kettle;
[0023] S2: According to the molar ratio of citric acid and formic acid is: 1:0.005-0.05, add formic acid catalyst in reactor;
[0024] S3: Add copper salt to the reaction kettle at normal temperature, the mass ratio of copper ions in citric acid and copper salt is 1:1.8-2.2, stir and react for 25-35min, filter, take the filtrate and put it into the reaction kettle, and heat up to 45 Stirring and reacting at -55°C for 25 minutes to generate citric acid chelated copper and dehydrate to obtain citric acid c...
Embodiment 1
[0029] A preparation method for high-purity citric acid chelated copper, comprising the following steps:
[0030] 1) Dissolve citric acid in desalinated water first, make a citric acid solution and put it into an enamel reaction kettle;
[0031] 2) add catalyst in reactor, catalyst is formic acid, according to the molar ratio of citric acid and described catalyst: 1:0.005;
[0032] 3) At room temperature, add basic copper carbonate to the reaction kettle, the mass ratio of citric acid and basic copper carbonate is 1:1, and stir for 30 minutes;
[0033] 4) Filter the reaction solution, take the filtrate and put it into the reaction kettle, heat up to 50°C, stir and react for 25min, and the reaction generates citric acid chelated copper;
[0034] 5) The product is centrifuged and dehydrated to obtain citric acid chelated copper crystals, and the liquid enters the mother liquor pool for recycling;
[0035] 6) using ethanol as a purifying agent to wash the obtained wet product c...
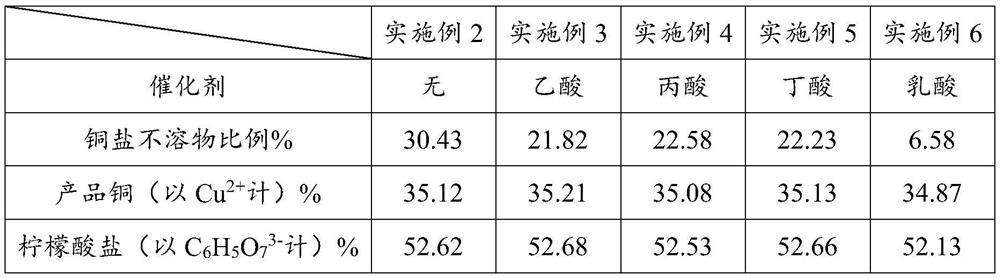
Embodiment 2
[0039] Embodiment 2 is compared with embodiment 1, except not adding formic acid catalyst, all the other are identical with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com