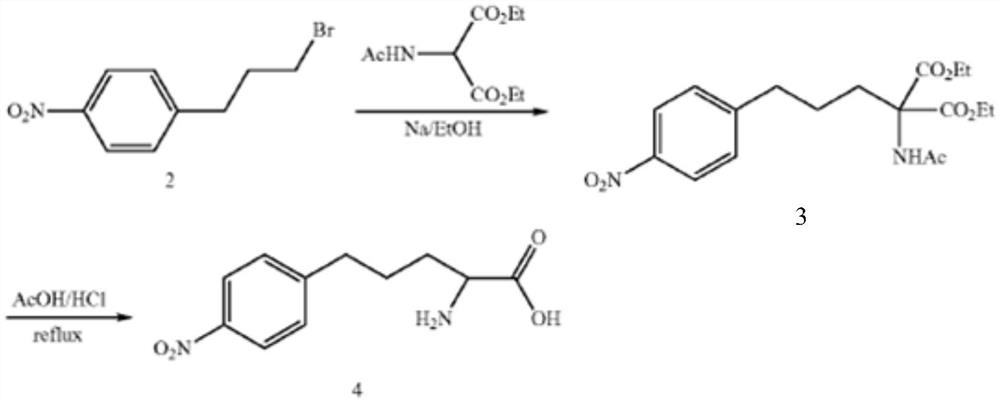
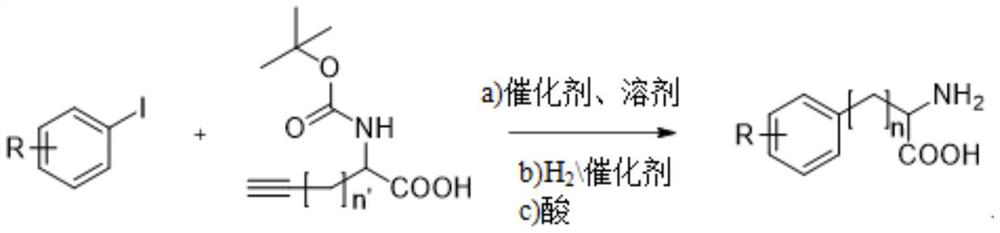
Synthesis method of multi-configuration long-chain phenyl amino acid compound
A technology of amino acids and synthetic methods, applied in the field of organic compound synthesis, can solve the problems of acetyl residue, low yield of final product compound 4, long reaction time and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
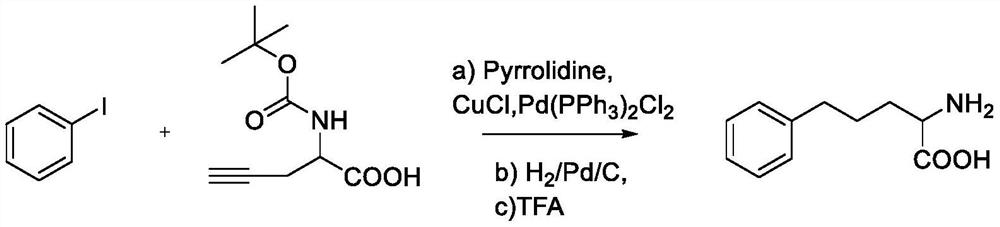
[0072] Synthesis of 2-amino-5-phenylpentanoic acid
[0073]
[0074] Under nitrogen, add iodobenzene (2.04g, 10mmol), N-tert-butoxycarbonyl-propargylglycine (2.13g, 10mmol), CuCl (0.04g, 0.40mmol), dissolve in 40mL tetrahydropyrrole, Then add Pd(PPh 3 ) 2 Cl 2 (0.14g, 0.2mmol), stirred at room temperature (25°C) for 1h, after the reaction, extracted with ethyl acetate, dried over anhydrous sodium sulfate, spin off most of the solvent, purified by silica gel column chromatography, using petroleum ether / Elution with ethyl acetate (V / V=15 / 1) gave 2-((tert-butoxycarbonyl)amino)-5-phenyl-4-pentynoic acid (2.6 g), which was then added with 10% Pd / C (1.16g) was dissolved in EtOH (15mL), THF (15mL) mixed solution, in H 2 After reacting for 2 hours at room temperature (25°C) under the conditions, after removing the solvent, add 20% (V / V) TFA / CH 2 Cl 2 (30 mL), the reaction mixture was stirred at room temperature (25°C) for 1 h, the aqueous phase was extracted three times with...
Embodiment 2
[0079] (R)-2-Amino-5-phenylpentanoic acid
[0080]
[0081] In this example, with iodobenzene (10mmol) and (R)-N-tert-butoxycarbonyl-propargylglycine (10mmol) as starting materials, the others are the same as in Example 1, and the final product (R)-2-amino- The yield of 5-phenylpentanoic acid was 89%.
[0082] Carry out NMR test to product, the result is as follows:
[0083] 1 H NMR (400MHz,D 2 O)δ7.26(m,3H),7.15(ddq,2H),3.83(tt,1H),2.55(tt,2H),1.82(m,2H),1.70(pd,2H).
[0084] 13 C NMR (125MHz,D 2 O) δ176.95(dd), 142.24(tt), 128.58(m), 126.41(tq), 56.70(ddd), 35.12(p), 30.83(td), 26.66(q).
Embodiment 3
[0086] (S)-2-Amino-5-phenylpentanoic acid
[0087]
[0088] In this example, with iodobenzene (10mmol) and (S)-N-tert-butoxycarbonyl-propargylglycine (10mmol) as starting materials, others are the same as in Example 1, and the final product (S)-2-amino- The yield of 5-phenylpentanoic acid was 90%.
[0089] Carry out NMR test to product, the result is as follows:
[0090] 1 H NMR (400MHz,D 2 O)δ7.26(m,3H),7.15(ddq,2H),3.83(tt,1H),2.55(tt,2H),1.82(m,2H),1.70(pd,2H).
[0091] 13 C NMR (125MHz,D 2 O) δ176.95(dd), 142.24(tt), 128.57(m), 126.41(tq), 56.94(ddd), 35.12(p), 30.84(ddd), 26.66(q).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap