New crystal form of cyclohexane formamide compound and preparation method thereof
A technology of compounds and crystal forms, applied in the field of medicinal chemistry, can solve problems such as differences in drug solubility, stability and mobile phase, affecting drug safety and effectiveness, and different clinical effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0128] (3) The preparation method of the crystal form provided by the present invention is simple and easy to operate and low in cost, and is suitable for drug research and development and industrial production.
[0129] Pharmaceutical compositions and methods of administration
[0130] Since the crystalline form or amorphous form of the present invention has excellent therapeutic and preventive effects on cancer or tumors, the crystalline form or amorphous form of the present invention and pharmaceutical compositions containing the crystalline form or amorphous form of the present invention as main active ingredients can be used For the treatment and / or prevention of anemia.
[0131] The pharmaceutical composition of the present invention comprises the crystal form of the present invention and pharmaceutically acceptable excipients or carriers within the safe and effective amount range.
[0132] Wherein, "safe and effective amount" refers to: the amount of the compound (or c...
Embodiment 1
[0145] Embodiment 1: Preparation of crystal form CM-I
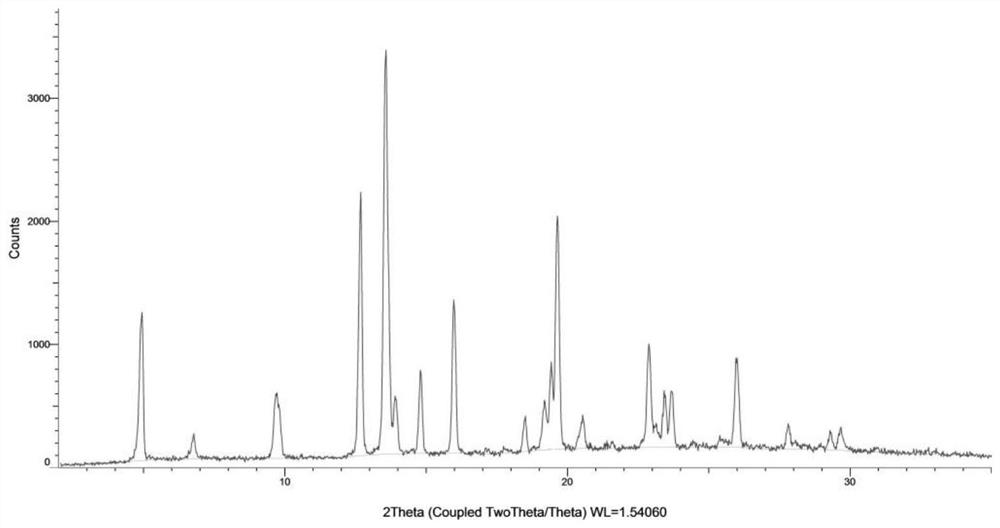
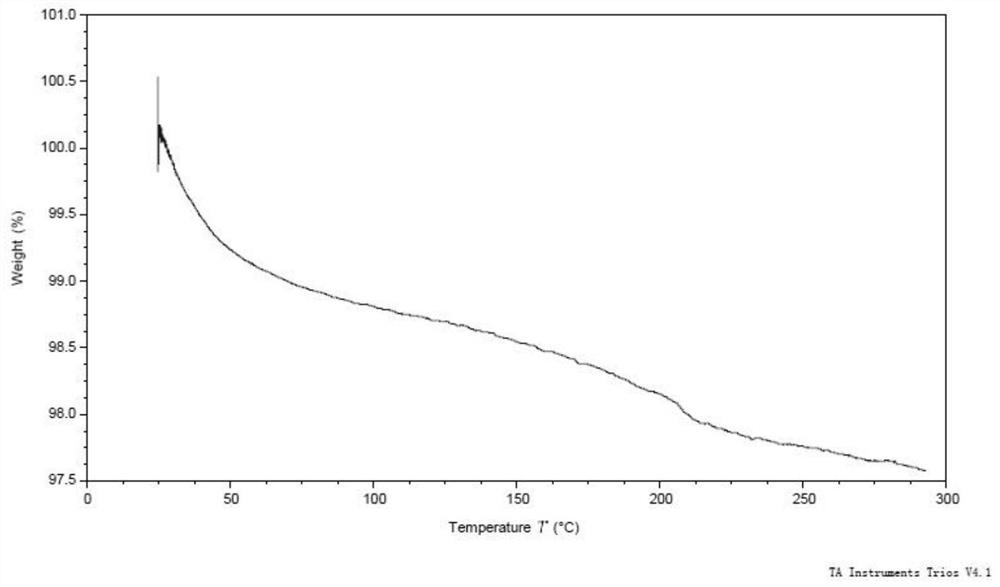
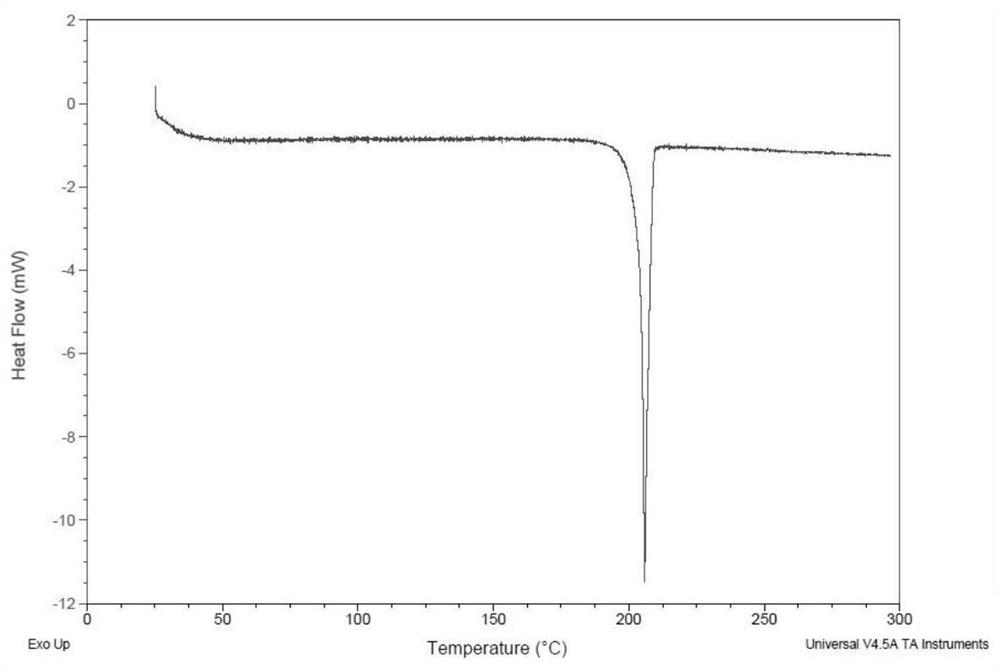
[0146] Weigh 14 mg of the compound of formula (I), dissolve it in 0.5 mL of ethanol at 30° C., and filter. The filtrate was placed at 5° C. and stirred for 24 h, and a solid precipitated out, which was the crystal form CM-I of the compound of formula (I). Carry out XRPD test to the obtained solid, its X-ray powder diffraction data are as shown in table 1, and its XRPD pattern is as follows figure 1 Shown; Carry out TGA test to gained solid, its spectrogram is as figure 2 Shown; Carry out DSC test to gained solid, its spectrogram is as image 3 shown; the obtained solid was 1 H NMR test, its spectrum is as Figure 4 As shown, NMR data: 1 H NMR (400MHz, DMSO-d 6 )δ11.89(s,1H),9.51(s,1H),8.68(d,J=4.1Hz,1H),8.45(dd,J=17.2,5.1Hz,2H),7.99(dd,J=8.6 ,2.2Hz,1H),7.89(dd,J=15.6,6.4Hz,2H),5.17–4.98(m,1H),3.13(s,3H),2.62(d,J=42.2Hz,1H),2.22 (d, J=13.8Hz, 6H), 2.10–1.52 (m, 9H), 1.46 (d, J=7.0Hz, 3H).
[0147] Table 1
[014...
Embodiment 2
[0149] Embodiment 2: Preparation of crystal form CM-II
[0150] Weigh 12 mg of the compound of formula (I), mix it with 0.2 mL of methanol, filter, and place the filtrate in a 3 mL open glass vial. Add 3 mL of methyl tert-butyl ether to a 20 mL large glass bottle. Put the 3mL glass vial containing the filtrate into the 20mL glass vial containing methyl tert-butyl ether, seal the 20mL glass vial, and let it stand at 25°C until a solid is precipitated, and the obtained solid is the compound of formula (I) Crystal form CM-II. Carry out XRPD test to the obtained solid, its X-ray powder diffraction data are as shown in table 2, and its XRPD figure is as follows Figure 9 Shown; Carry out TGA test to gained solid, its spectrogram is as Figure 10 Shown; Carry out DSC test to gained solid, its spectrogram is as Figure 11 shown; the obtained solid was 1 H NMR test, its spectrum is as Figure 12 As shown, NMR data: 1 HNMR (400MHz, DMSO-d 6 )δ11.89(s,1H),9.52(s,1H),8.68(d,J=4.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com