Selenium polymer prodrug micelle with reduction responsiveness, preparation method and application
A polymer, responsive technology that can be used in the field of biomedicine to solve problems such as limiting mass production applications, synthesis process complexity, and non-reproducibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] The preparation method of the above-mentioned reduction-responsive selenium polymer prodrug micelles comprises the following steps:
[0057] S1, under a protective gas atmosphere, using ethyl 7-bromoheptanoate and sodium perselenide as raw materials to synthesize the compound of formula (Ⅲ);
[0058] S2, the compound of formula (Ⅲ) is deesterified under alkaline conditions to generate the compound of formula (Ⅳ);
[0059] S3. Under a protective gas atmosphere, dissolve the compound of formula (IV), 3-aminobenzyl alcohol, HOAT, and HATU in a solvent, add N,N-diisopropylethylamine (DIEA) at 0°C, and generate the formula ( V) compounds;
[0060] S4, reacting the compound of formula (V) and carbonyldiimidazole to prepare the compound of formula (VI);
[0061] S5, under protective gas atmosphere, formula (Ⅵ) compound and amino polyethylene glycol monomethyl ether (mPEG 2000 -NH 2 ,M w =2.0KDa) reaction, replace and prepare the selenium polymer of formula (I); self-assem...
Embodiment 1
[0066] A method for preparing a reduction-responsive selenium polymer prodrug micelle, comprising the following steps:
[0067] 1) Preparation of 7,7'-diselenideethyl heptanoate from ethyl 7-bromoheptanoate;
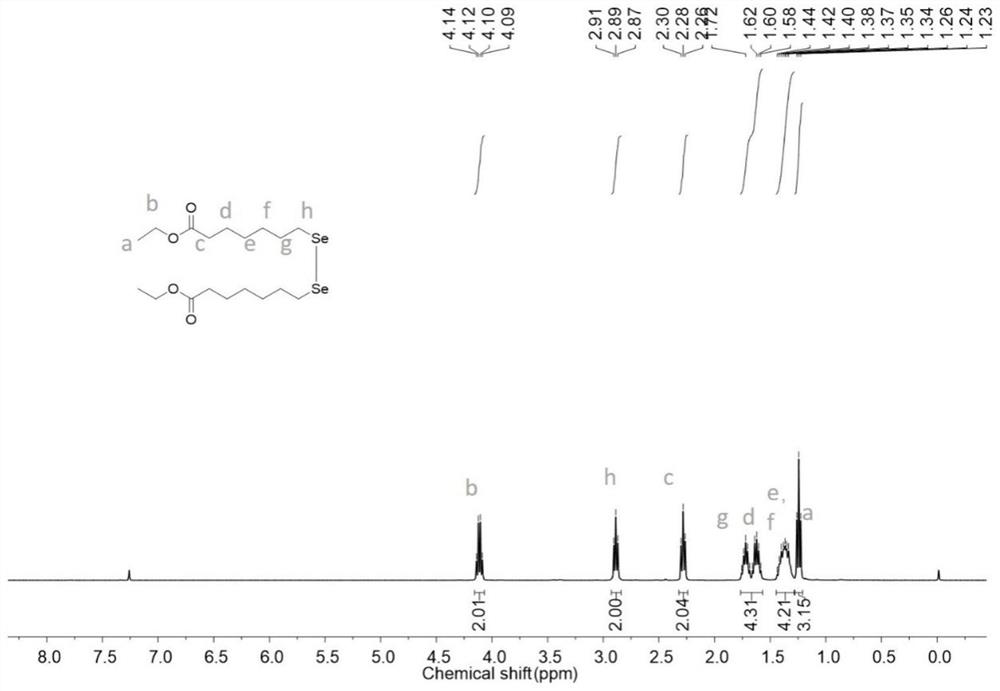
[0068] The compound 7-bromoheptanoic acid ethyl ester (2.42g, 13.87mmol) was added into the reaction flask, tetrahydrofuran (15mL) was added under nitrogen protection, and sodium perselenide (6.94mmol) was added at the same time; reacted at 40°C for 8h, returned to room temperature, and spun Dry tetrahydrofuran, extract the aqueous phase with ethyl acetate (30mL×3) three times, combine the organic phases and dry with anhydrous sodium sulfate, filter with suction, and spin to dry the solvent to obtain 1.68g of light yellow oil, yield: 74%; figure 1 The obtained 7,7'-diselenide ethyl heptanoate NMR pattern is given 1 H NMR;
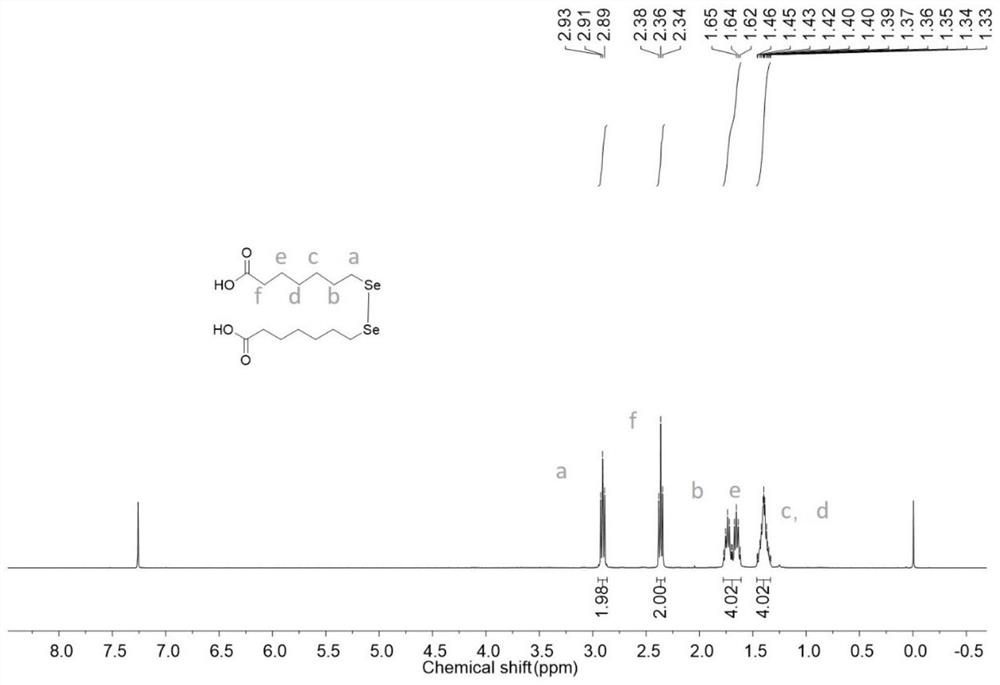
[0069] 2) preparing 7,7'-diselenideptanoic acid from ethyl 7,7'-diselenideptanoate;
[0070] Compound 7,7'-diselenide ethyl heptanoate (4.00g, 8.8...
Embodiment 2
[0078] A method for preparing a reduction-responsive selenium polymer prodrug micelle, comprising the following steps:
[0079]1) Preparation of ethyl 7,7'-diselenidediheptanoate from ethyl 7-bromoheptanoate, the steps are the same as in Example 1, the difference is that the reaction at 25°C for 12h, ethyl 7-bromoheptanoate and perselenium The molar ratio of sodium chloride is 3:1;
[0080] 2) Preparation of 7,7'-diselenide heptanoate from ethyl 7,7'-diselenide diheptanoate, the steps are the same as in Example 1, the difference is that the reaction time is 2h, 7,7'-diselenide diheptanoate The mol ratio of ethyl diheptanoate and sodium hydroxide is 1:2;
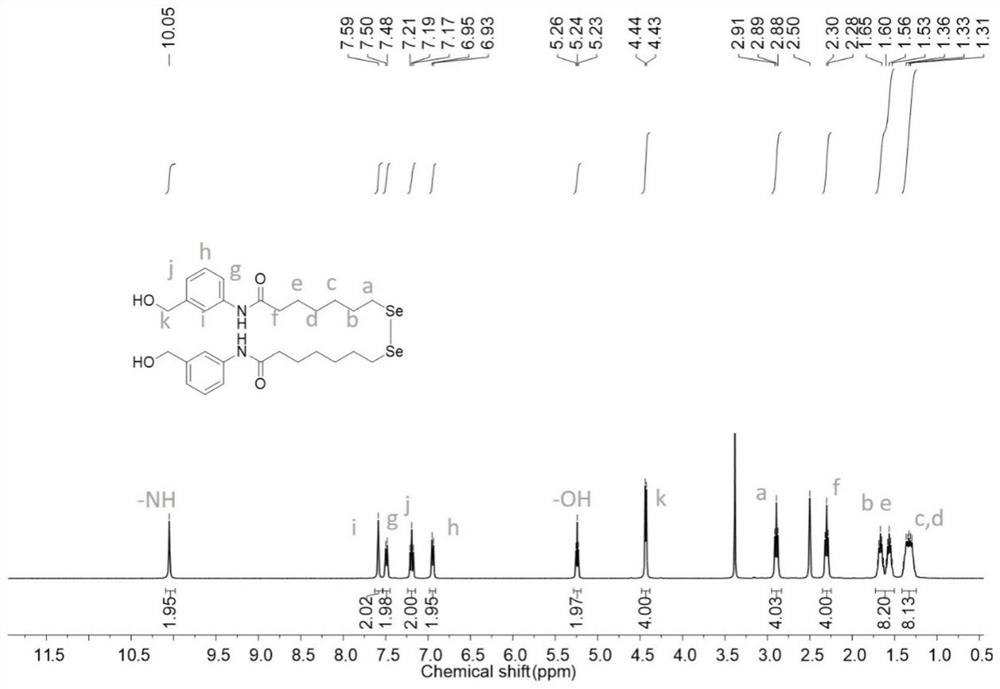
[0081] 3) Prepare DmSeSAHA from 7,7'-diselenideheptanoic acid, the steps are the same as in Example 1, the difference is that the reaction temperature is 25°C, 7,7'-diselenideheptanoic acid: 3-aminobenzyl alcohol: The molar ratio of HATU:HOAT:DIEA is 1:4:4:4:4;
[0082] 4) Prepare CDI-DmSeSAHA from DmSeSAHA, the steps are ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com