Preparation method of high-modulus high-thermal-conductivity polyimide film
A polyimide film, polyamic acid technology, applied in the direction of semiconductor/solid-state device manufacturing, semiconductor devices, semiconductor/solid-state device components, etc. Conductivity improvement is limited and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
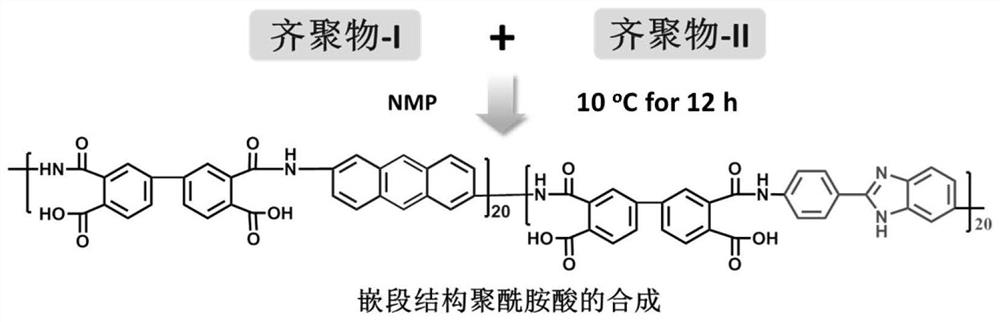
[0042](1) Under nitrogen protection, in a three-necked flask, add 50mL of NMP, 2.76g (0.0133mol) of anthracene-2,6-diamine (Beijing Dingsheng Brothers Technology Co., Ltd., purity 99%), 4.12g ( 0.014mol) of 3,3',4,4'-biphenyltetraacid dianhydride (BPDA) (Changzhou Sunshine Pharmaceutical Co., Ltd., 99.5%), fully stirred and reacted for 12 hours, and controlled the reaction temperature at 2°C to obtain anhydride-terminated polymer Amic acid oligomer-I solution. Under nitrogen protection, in a three-necked flask, successively add 50 mL of NMP, 2.98 g (0.0133 mol) of 5-amino 2-(4-aminophenyl)-benzimidazole (BIA) (Changzhou Sunshine Pharmaceutical Co., Ltd., Purity 99%), 3.70g (0.0126mol) of 3,3',4,4'-biphenyl tetraic acid dianhydride (BPDA) (Changzhou Sunshine Pharmaceutical Co., Ltd., 99.5%), fully stirred for 12 hours, and controlled the reaction temperature to At 2°C, an amino-terminated polyamic acid oligomer-II solution was obtained. Stir and mix the oligomer-I solution an...
Embodiment 2
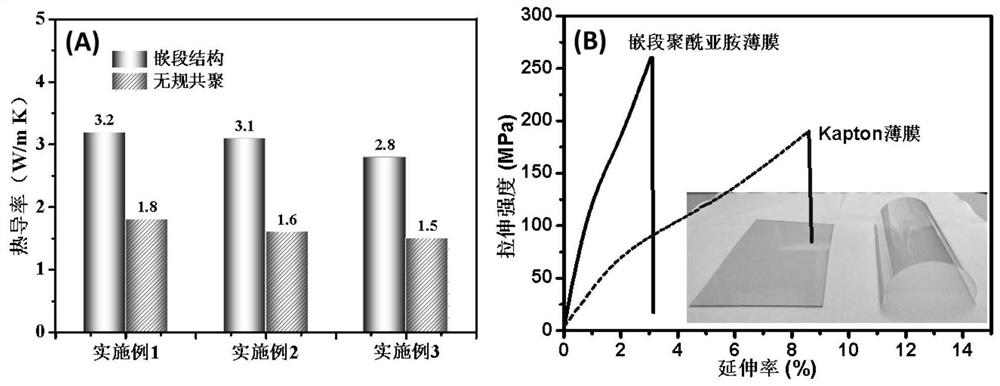
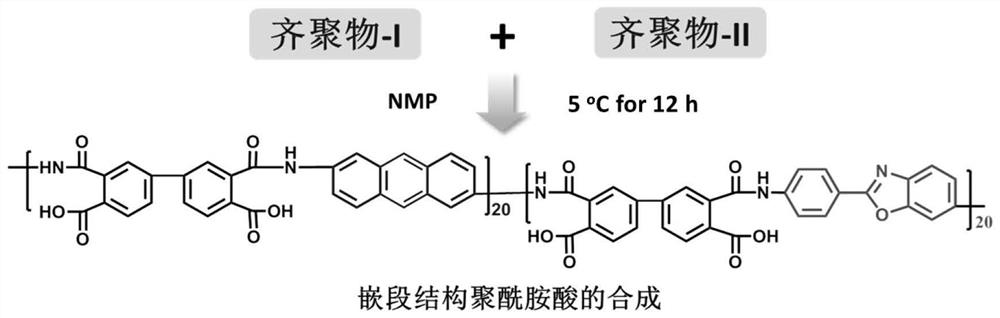
[0046] (1) The preparation of the anhydride-terminated polyamic acid oligomer-I solution is the same as in Example 1. According to Example 1, change "2.98g (0.0133mol) of 5-amino 2-(4-aminophenyl)-benzimidazole (BIA)" to "2.99g (0.0133mol) of 5-amino 2-( 4-aminophenyl)-benzoxazole (BOA) (Changzhou Sunshine Pharmaceutical Co., Ltd., purity 99%)", and the rest are the same as in Example 1 to obtain amino-terminated polyamic acid oligomer-II solution. Stir and mix the oligomer-I solution and the oligomer-II solution in a nitrogen atmosphere, and react at 5°C for 12 hours to obtain a block-structured polyamic acid precursor solution with an apparent viscosity of 4980cP·s (25°C) , molecular structure such as image 3 shown.
[0047] (2) The polyamic acid precursor solution in step (1) was cast into a film under the same conditions as in Example 1 to obtain a cured polyamic acid precursor film. Continue thermal cyclization and bidirectional thermal drawing treatment on the cured ...
Embodiment 3
[0050] (1) According to Example 1, change "2.76g (0.0133mol) of anthracene-2,6-diamine" to "3.16g (0.0133mol) of 2,6-diamino-anthraquinone (Cobant Chemical Industry (Hangzhou) Co., Ltd., purity 99%) ", all the other are identical with embodiment 1, obtain anhydride-terminated polyamic acid oligomer-I solution. According to Example 1, change "2.98g (0.0133mol) of 5-amino 2-(4-aminophenyl)-benzimidazole (BIA)" to "2.99g (0.0133mol) of 5-amino 2-( 4-aminophenyl)-benzoxazole (BOA) (Changzhou Sunshine Pharmaceutical Co., Ltd., purity 99%)", and the rest are the same as in Example 1 to obtain amino-terminated polyamic acid oligomer-II solution. Stir and mix the oligomer-I solution and the oligomer-II solution in a nitrogen atmosphere, and react at 5°C for 12 hours to obtain a block-structured polyamic acid precursor solution with an apparent viscosity of 4280cP·s (25°C) , molecular structure such as Figure 4 shown.
[0051] (2) The polyamic acid precursor solution in step (1) wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com