Polypeptide, HLA-DR protein and preparation method and application of HLA-DR protein
An HLA-DR, protein technology, applied in the field of polypeptide, HLA-DR protein and its preparation, can solve the problems of low yield, low yield, uncontrollable and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
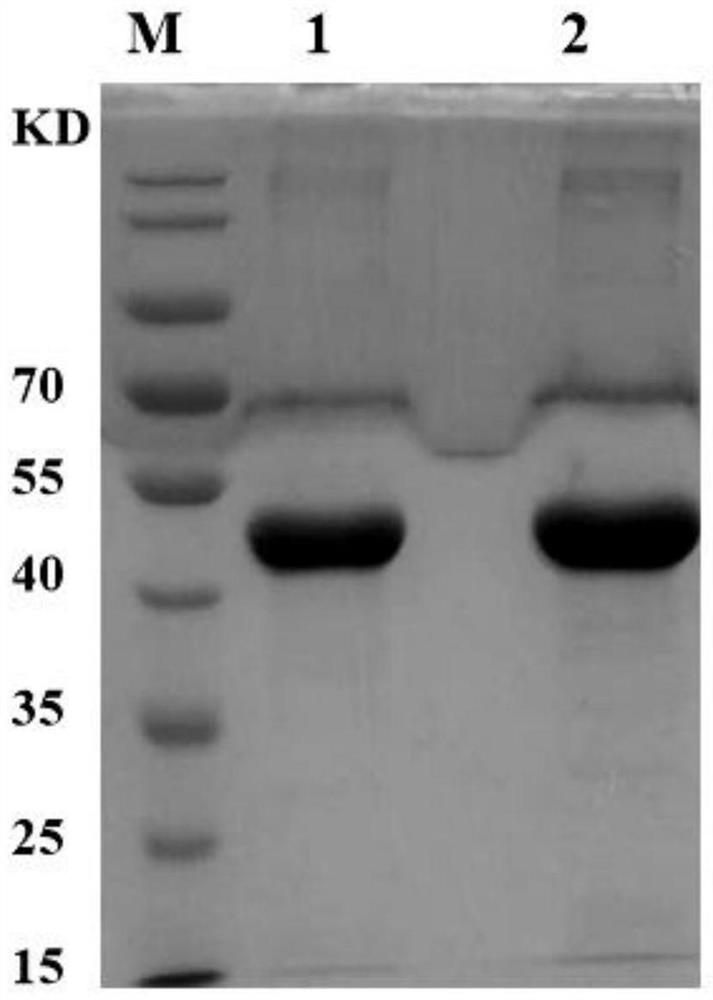
[0087] Example 1 HLA-DR protein preparation method (taking SEQ ID NO: 13 as an example)
[0088] 1. Carrier construction
[0089] 1.1. Acquisition of α chain amino acid sequence
[0090] The α chain amino acid sequence and gene sequence of human human leukocyte antigen-DR (HLA-DR) were obtained from the NCBI database (UniProtKB-P01903(DRA_HUMAN); Gene ID: 3122, updated on 16-Feb-2020); HLA - The nucleotide sequence of DRα chain is shown in SEQ ID NO:49.
[0091] 1.2. Acquisition of β chain amino acid sequence
[0092] The β chains are mainly DRB1, DRB3, DRB4, and DRB5, among which DRB1 has the most variation. According to the uniprot database, 50 DRB1, 24 DRB3, 19 DRB4, and 22 DRB5 protein sequences were screened; further comparison and screening were performed to obtain sequences with higher homology, and the β-chain amino acid sequence was designed, with a total of 231 amino acids.
[0093] According to the amino acid sequence of the β chain, the nucleotide sequence of t...
Embodiment 2
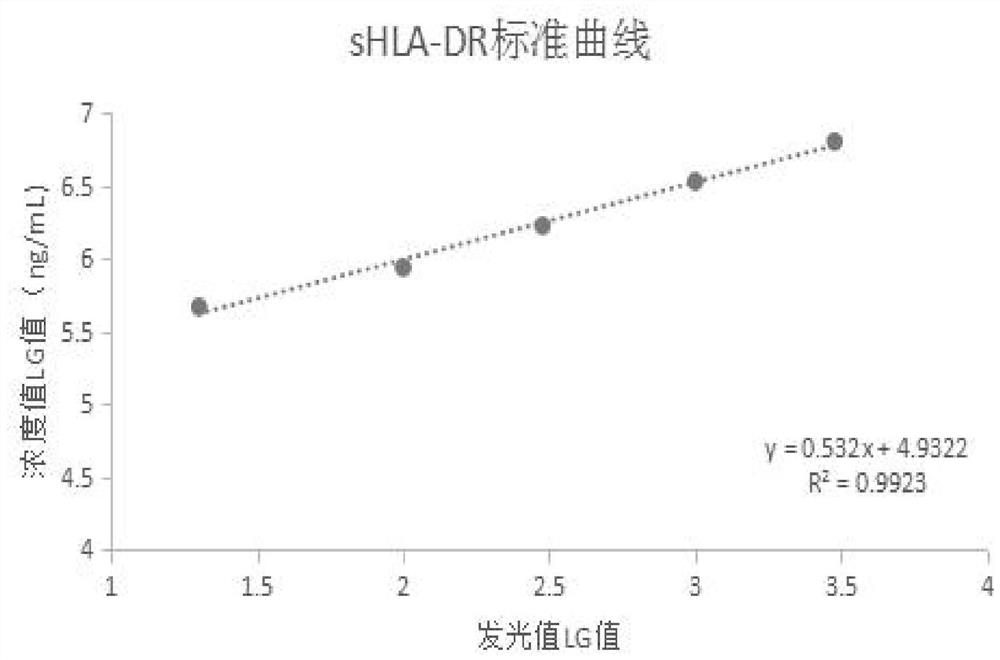
[0121] Example 2 Principle and detection steps of sHLA-DR quantitative detection kit
[0122] 1. Inspection principle
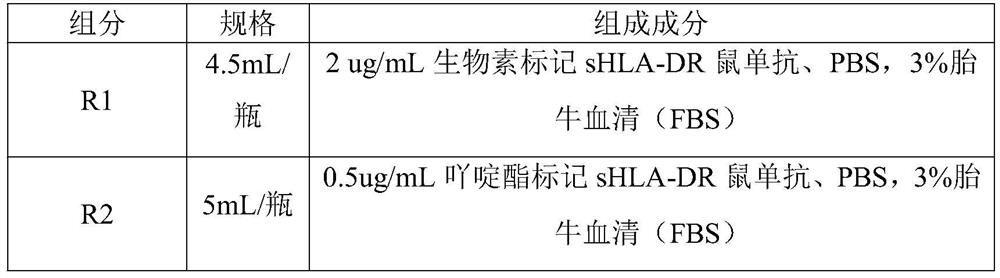
[0123] The quantitative detection kit uses the double-antibody sandwich method to detect the content of soluble human leukocyte antigen-DR (sHLA-DR) in plasma. The kit uses a pair of monoclonal antibodies against sHLA-DR (commercially available), one of which is labeled with biotin, and the other is labeled with acridinium ester. The specimen / calibration solution / quality control solution reacts with biotin-labeled monoclonal antibody, acridinium ester-labeled monoclonal antibody, and immunomagnetic particles coated with streptavidin to form an immune complex. The amount of the complex formed It is directly proportional to the content of the antigen to be tested. The acridinium ester in the complex releases photons under the excitation of the substrate solution, automatically monitors the relative light intensity RLU emitted within 3 seconds, and has a certa...
Embodiment 3
[0141] Example 3 Effect of Linker Length Changes on Fusion Protein Activity
[0142] In this embodiment, 6 groups of Linkers with different lengths are designed, as shown in Table 3 below:
[0143] table 3:
[0144]
[0145] Table 4:
[0146]
[0147] The luminescence value in Table 4 is the luminescence value of the sample in the quantitative detection system by diluting the target protein obtained by different design schemes in Table 3 into different concentrations (10000ng / mL, 5000ng / mL, etc.); the membrane protein is the control group. The closer the luminescence value of the isoconcentration sample is to the membrane protein, the better the protein activity; the protein activity is evaluated by calculating the average value of the ratio of the luminescence value of the isoconcentration sample to the membrane protein luminescence value.
[0148] From the above experimental data, it can be seen that when the length of the Linker is 8-48 amino acids, the protein acti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com