Preparation method of human neuregulin 4
A technology for regulating protein and human nerve, applied in the field of preparation of human neuregulin 4, which can solve the problems of low yield and no biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1 Optimization and synthesis of human neuregulin 4 gene
[0074] According to the human neuregulin 4 gene sequence reported in the literature (GenBank: CAL35829.1), the gene expression sequence of the non-transmembrane region was optimized. The optimized sequence is shown in the sequence table SEQ.1, the amino acid sequence of the encoded human neuregulin 4 See the sequence listing SEQ.2. Synthesize 8 complementary oligonucleotides, according to the conventional method of molecular cloning, first treat with T4 bacteriophage polynucleotide kinase at 37°C for 30min, mix the phosphorylated oligonucleotide fragments in equimolarity, denature at 94°C for 5min, immediately Anneal at 65°C for 10 minutes, then add T4 ligase, and ligate overnight at 16°C to obtain target gene template fragments. Take 4 sterile microcentrifuge tubes and add:
[0075]
[0076] The above mixture was shaken gently to mix well, then briefly centrifuged, and then placed in a water bath at...
Embodiment 2
[0077] Example 2 Cloning and expression of SUMO protein-human neuregulin 4 fusion gene
[0078] Design a pair of primers for gene amplification, and connect with the gene sequence of SUMO protein to obtain the fusion protein gene. See sequence listing SEQ.3. Then design the gene 5' end primer with Nde I restriction site, and the 3' end primer with stop codon and Bam H I restriction site.
[0079] The gene amplification of human neuregulin 4 was carried out as follows, and a 25 μl reaction system was prepared in a 0.2ml PCR microcentrifuge tube:
[0080]
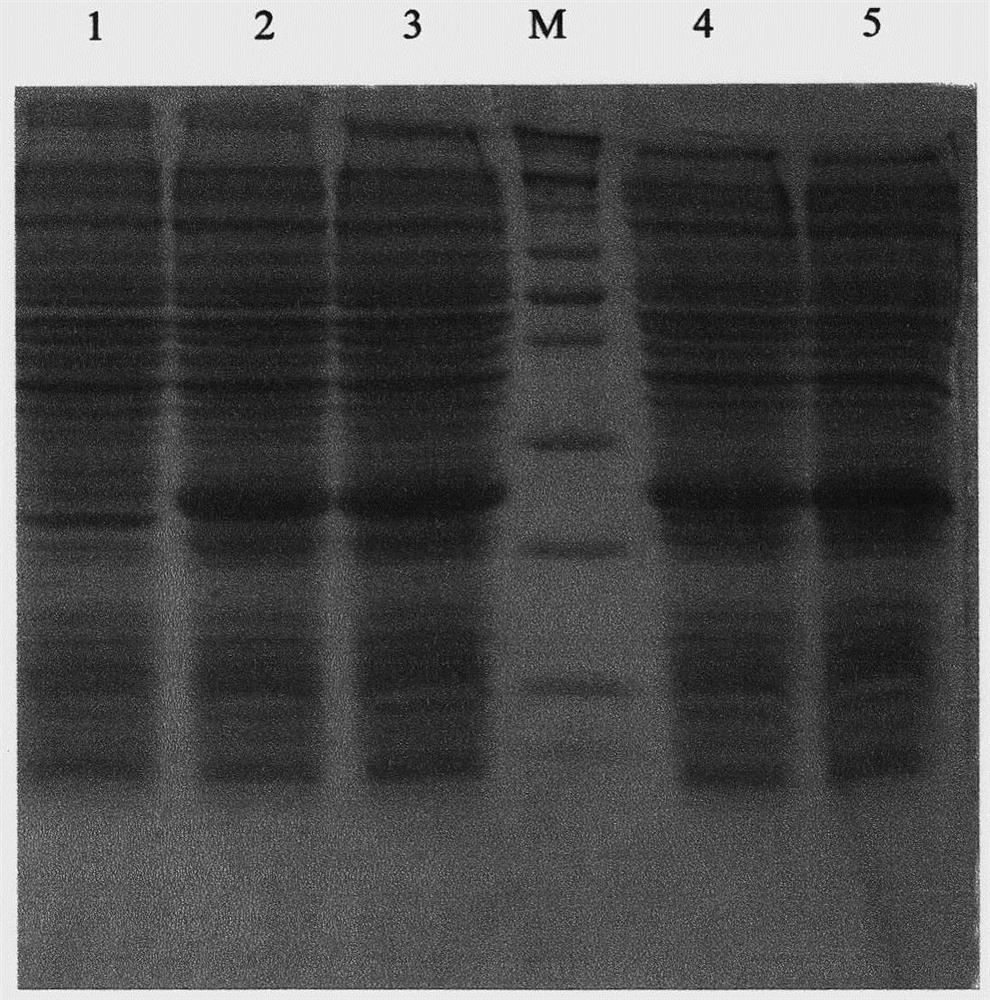
[0081] Use a small pipette tip to stick the plasmid template to the selected white colony, then insert it into the PCR mixture and wash it. The amount of Taq enzyme added is 2U. Pre-denatured at 94°C for 5 minutes, set 94°C for 1min, 56°C for 1min, 72°C for 2min, a total of 35 cycles, and finally 72°C for 15min. After PCR, 10 μl of the product was taken for agarose gel electrophoresis. The fragment size was consistent w...
Embodiment 3
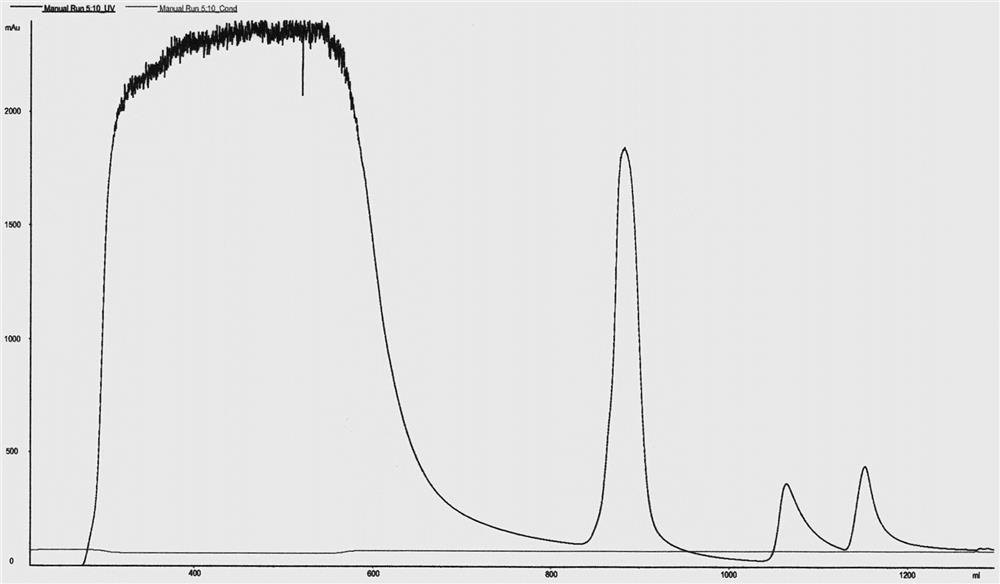
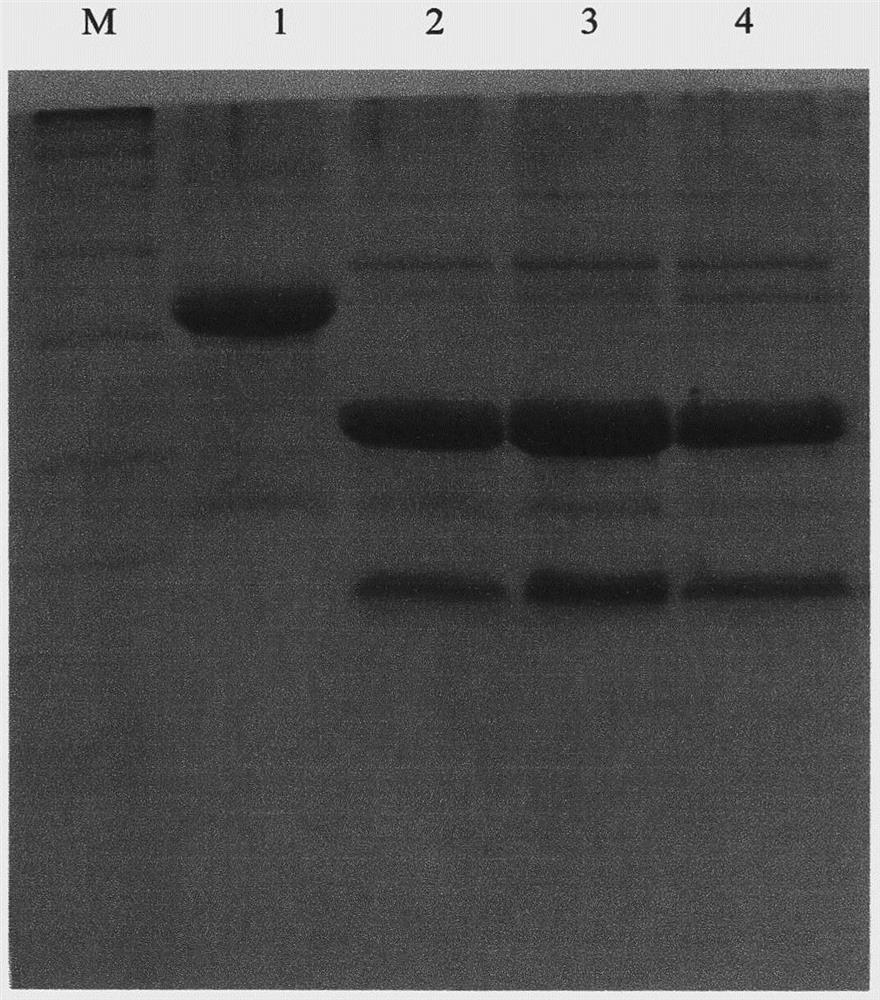
[0085] Example 3 Separation and purification of SUMO protein-human neuregulin 4 fusion protein
[0086] 1. Bacteria culture
[0087] Prepare 1 liter of LB medium (tryptone 10g / L, yeast extract 5g / L, sodium chloride 10g / L, pH 7.0), and after the medium is prepared, sterilize it at 121°C for 40 minutes. After the sterilization, add Amp to the ultra-clean bench to make the final concentration of 50 μg / mL when the culture medium is cooled and not hot, and put it in the refrigerator at 4 °C after cooling for later use.
[0088] Pick a single colony of engineering bacteria containing the plasmid pET-32a-SUMO-NRG4 from the plate under aseptic conditions and put it in 100ml LB medium (containing Amp), then place it at 37°C and culture overnight on a shaker at 250rpm. Transfer the overnight seed culture to LB medium at 0.5% inoculum, cultivate at 37°C and 250rpm for 4 hours, the OD600 of the culture medium is about 0.6-0.8, and then add the inducer IPTG to a final concentration of 1mm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com