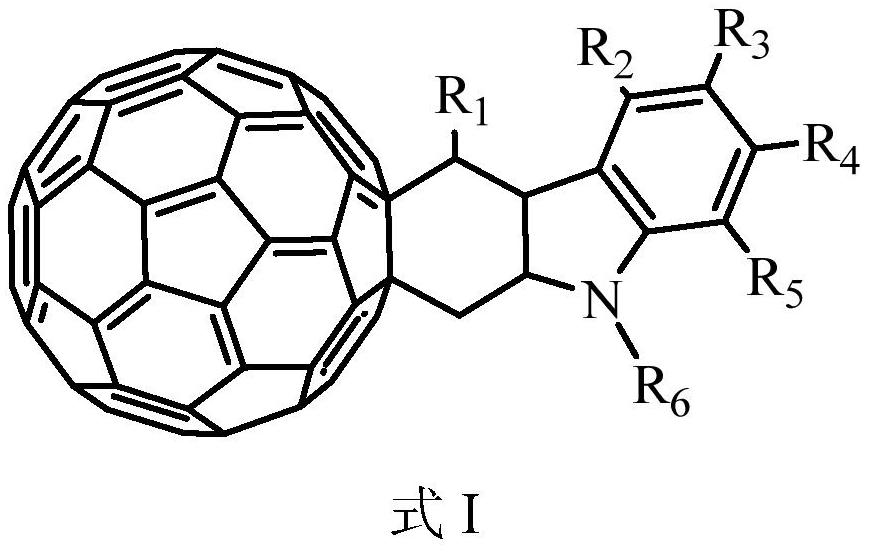
Organic photoelectric material containing C60 condensed ring, preparation method and application thereof
A technology of organic photoelectric materials and condensed rings, which is applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of high cost, poor stability, and many steps of axenic compounds, and achieve improved film-forming performance and electrical conductivity. The effect of increasing the efficiency and improving the hole injection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
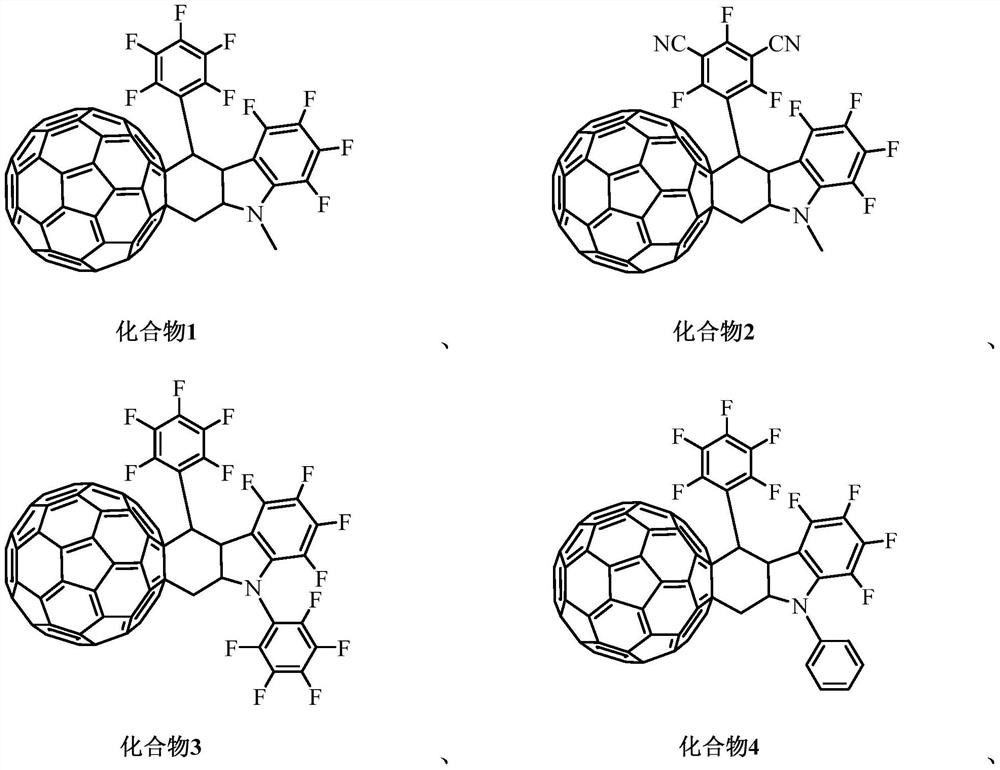
[0048] This embodiment provides an organic optoelectronic material compound 1 containing a C60 fused ring, its structural formula is as follows The reaction formula for preparing compound 1 is:
[0049]
[0050] Concrete preparation method comprises the following steps:
[0051] Add 0.49g of fullerene C60 (1mmol), 0.40g of compound 1a (2mmol), 0.16g of 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ, 0.7mmol) and 5mL of chlorobenzene into the reaction flask , and reacted at 120°C for 10 hours; filtered and concentrated to obtain a crude product; then separated and purified with a 200-300 mesh silica gel column to obtain the product compound 1.
[0052] Characterization data: mass spectrum MS: 887;
[0053] NMR 1 H-NMR (400 MHz, CDCl 3 ): δ1.52(d,2H), 2.85(s,3H), 2.99(m,1H), 3.32(d,1H), 3.37(t,1H).
Embodiment 2
[0055] This embodiment provides an organic photoelectric material compound 2 containing a C60 fused ring, its structural formula is as follows The reaction formula for preparing compound 2 is:
[0056]
[0057] Concrete preparation method comprises the following steps:
[0058] Add 0.49g of fullerene C60 (1mmol), 0.82g of compound 2a (2mmol), 0.16g of 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ, 0.7mmol) and 5mL of chlorobenzene into the reaction flask , and reacted at 120°C for 10 hours; filtered and concentrated to obtain a crude product; then separated and purified with a 200-300 mesh silica gel column to obtain the product compound 2.
[0059] Characterization data: mass spectrum MS: 901;
[0060] NMR 1 H-NMR (400 MHz, CDCl 3 ): δ1.52(d,2H), 2.85(s,3H), 2.99(m,1H), 3.36(d,1H), 3.40(t,1H).
Embodiment 3
[0062] This embodiment provides an organic photoelectric material compound 3 containing a C60 fused ring, its structural formula is as follows The reaction formula for preparing compound 3 is:
[0063]
[0064] Concrete preparation method comprises the following steps:
[0065] Add 0.49g of fullerene C60 (1mmol), 1.10g of compound 3a (2mmol), 0.16g of 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ, 0.7mmol) and 5mL of chlorobenzene into the reaction flask , and reacted at 120°C for 10 hours; filtered and concentrated to obtain a crude product; then separated and purified with a 200-300 mesh silica gel column to obtain the product compound 3.
[0066] Characterization data: mass spectrum MS: 1039;
[0067] NMR 1 H-NMR (400 MHz, CDCl 3 ): δ1.52(d,2H), 2.99(m,1H), 3.32(d,1H), 3.37(t,1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com