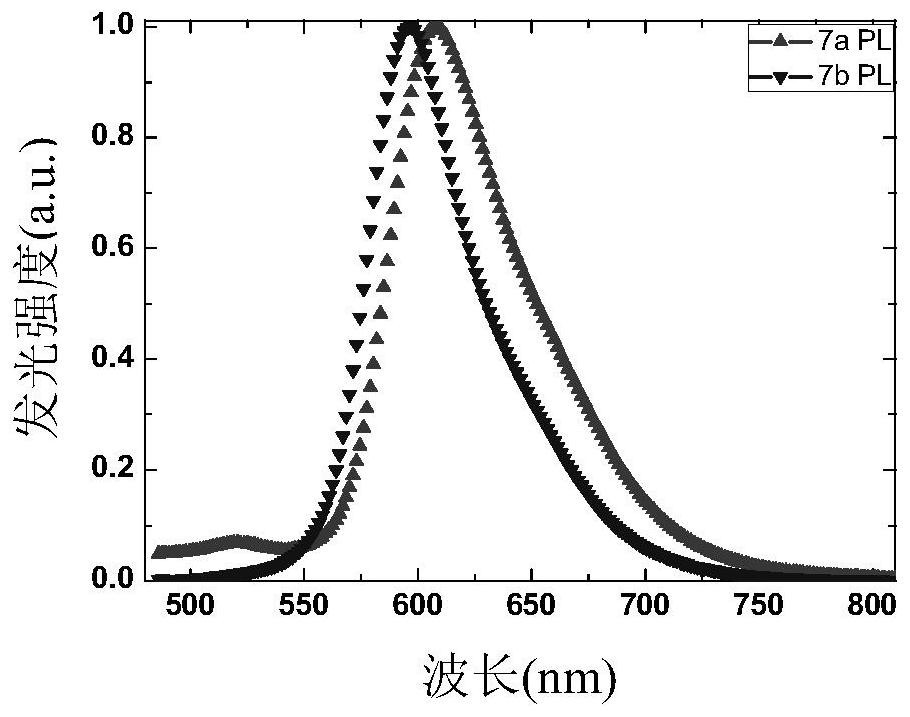
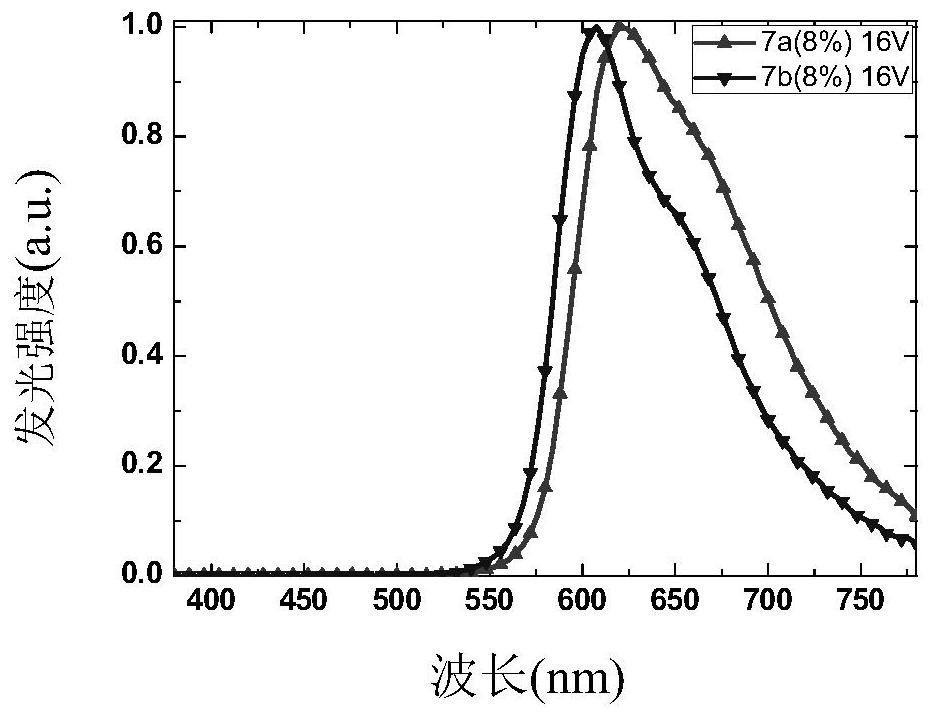
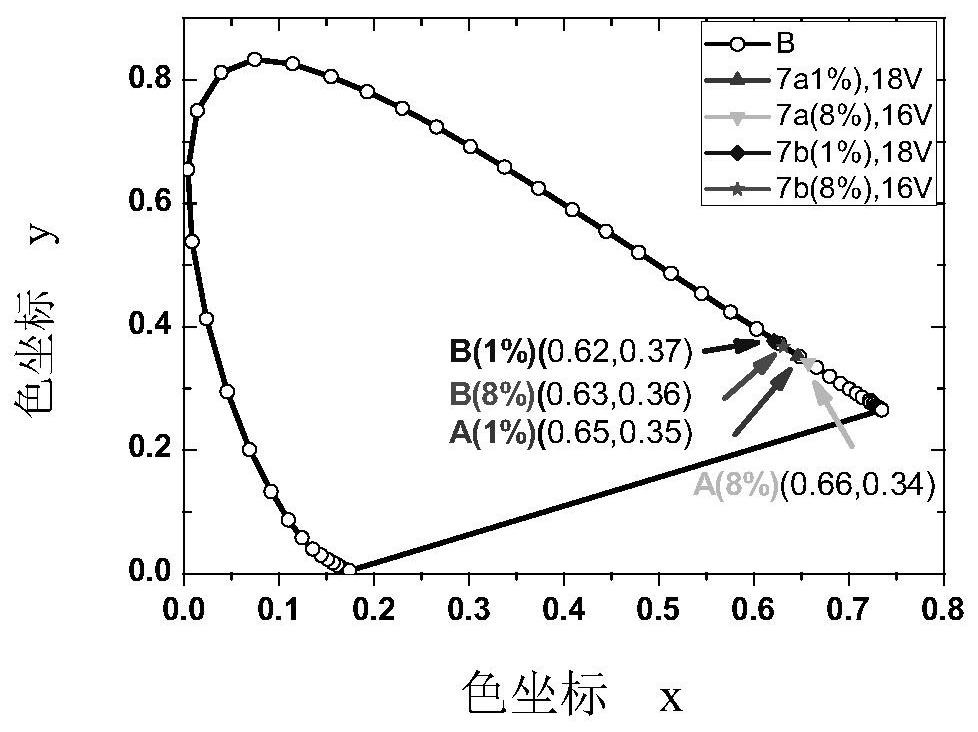
Fluorenyl phenylquinoline iridium complex with saturated red luminescence
A technology of fluorenylbenzoquinoline iridium and red light emission, which is applied in the direction of light-emitting materials, indium organic compounds, platinum group organic compounds, etc., and can solve problems such as inconvenient operation and complicated process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0083] Example 1 Bis[2-(4-(N-9,9-diethylfluorene-N-3,5-xylanilino)phenyl)-4-phenylquinoline-C 2 ,N]iridium (2-pyridone) ((FPAPPQ) 2 Synthesis of Ir(acac)(7a)):
[0084] (1) Under argon protection, add 3,5-xylaniline (2.95g, 24.37mmol), 2-bromo-9,9-diethylfluorene (5.91g, 19.6mmol) into a 250ml single-necked round bottom flask ), Na-O-t-Bu (2.638g, 27.45mmol) and toluene, put into a magnet, stir and dissolve, fill a 50ml single-necked round bottom flask with argon (Ar) for 3 times, then add Pd(AcO) 2 (0.044g, 0.195mmol), X-phos (0.28g, 0.587mmol), toluene, after being dissolved, quickly added to the above reaction solution, filled with argon (Ar) twice, sealed with liquid paraffin, magnetically stirred, heated Temperature 110°C, 20h reaction, ethyl acetate extraction, drying, concentration, silica gel column separation, 2-(3,5-xylanilino)-9,9-diethylfluorene (fpa) white solid, yield 85.3%;
[0085] (2) Take a 250ml round bottom flask, put it into a magnet, weigh 1.974g (5.4...
Embodiment 2
[0091] Example 2: Bis[2-(4-(N-9,9-diethylfluorene-N-3,5-xylanilino)phenyl)-4-phenylquinoline-C 2 ,N]iridium (2-pyridine acid) ((FPAPPQ) 2 Synthesis of Ir(pic)(7b))
[0092] Steps (1)-(3) are the same as in Example 1;
[0093] (4) Take 0.44g intermediate [Ir(ppq) 2 Cl] 2 , 0.067g of sodium carbonate and pyridine acid (0.051g) were dissolved in ethylene glycol monomethyl ether (14ml), and refluxed for 20 hours under argon protection. After cooling, the mixture was separated through a silica gel column to obtain an orange-yellow solid (FPAPPQ) 2 Ir(pic)(7b)), yield 71.8%.
[0094] Product Confirmation:
[0095] 1 H NMR (400MHz, CDCl 3 )–δ(ppm):0.34–0.38(t,12H),1.90–2.01(m,8H),2.26(s,12H),6.73–7.07(m,12H),7.25–7.31(m,8H), 7.42–7.61(m,20H),7.73(d,2H),7.75-8.42(m,4H),8.56(d,2H),8.36–8.38(d,1H),9.11(s,1H);
[0096] Elemental analysis (molecular formula C 98 h 82 IrN 5 o 2 ): theoretical value: C, 75.75; H, 5.32; N, 4.51, tested value: C, 75.39; H, 5.28; N, 4.42.
Embodiment 3 2
[0097] Example 3 Bis[2-(4-(2-diethylfluorenyl)phenyl)-4-phenylquinoline-C 2 ,N]iridium(pentanedione)((FPPQ) 2 Synthesis of Ir(acac)(5a)):
[0098] (1) Dissolve 2-bromo-9,9'-diethylfluorene (30mmol, 9.0g) in 200ml tetrahydrofuran in a round-bottomed flask, and cool to -78°C under the protection of argon; add n-butyl dropwise Lithium base (1.6M, 45mmol, 28.1ml) was reacted for 1 hour, trimethyl borate (45mmol, 8.83g) was added once; the reaction was raised to room temperature for 30 minutes, and then reacted for 1 hour; treated with dilute acid and extracted with ethyl acetate , dried, concentrated, and separated by silica gel column chromatography to obtain a white solid 9,9'-diethylfluorene-2-boronic acid with a yield of 52.3w%;
[0099] (2) Under the protection of argon, 2-(4-bromophenyl)-4-phenylquinoline (2.484g, 6.90mmol), 9,9'-diethylfluorene-2-boronic acid (2.02g, 7.58mmol), dissolved in 36ml of anhydrous toluene and 24ml of absolute ethanol, adding 2M Na 2 CO 3(3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com