Ganoderma quality evaluation method based on in-vitro tumor cell culture model
A technology of tumor cells and Ganoderma lucidum, applied in biochemical equipment and methods, measuring devices, and microbial determination/inspection, etc., can solve the problem of lack of microenvironment for cell growth and differentiation, and can not simulate tumor cells and tumor microenvironment well Interaction, the correlation between the clinical application of Ganoderma lucidum and its curative effect is not strong, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Evaluation of the quality of Ganoderma lucidum based on chemical detection
[0060] Five batches of Ganoderma lucidum and five batches of Ganoderma sinense were provided by Wuyi Shouxiangu Traditional Chinese Medicine Pieces Co., Ltd. According to "Chinese Pharmacopoeia" 2015 edition, the method stipulated under [Content Determination] under Ganoderma lucidum item, the content of polysaccharides, triterpenes and sterols of these 10 batches of Ganoderma lucidum samples were determined: the determination of polysaccharides was carried out by anthrone-sulfuric acid method, Anhydrous glucose was used as the reference substance; triterpenes and sterols were determined by the vanillin-glacial acetic acid method, and oleanolic acid was used as the reference substance. The test results are shown in Table 1.
[0061] It can be seen from Table 1 that the polysaccharide content of 5 batches of Chizhi is 1.19%-1.91%, the content of triterpenes and sterols is 0.85%-1.58%...
Embodiment 2
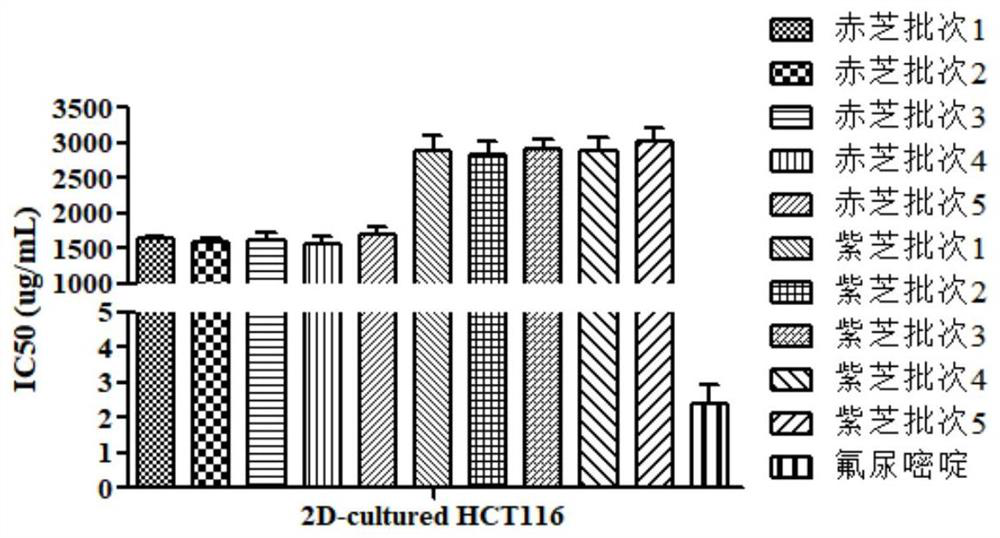
[0064] Example 2: Quality evaluation of Ganoderma lucidum based on 2D tumor cell culture model
[0065] Get the same red sesame and purple sesame medicinal materials as in Example 1, and evaluate its quality according to the following steps:
[0066] (1) Take 2g each of different batches of ganoderma lucidum medicinal powder (passed through a 40-mesh sieve), add 50mL of water, heat and reflux in a water bath for 1 hour, filter, put the filtrate on a water bath and evaporate to dryness, and obtain a ganoderma lucidum sample to be tested;
[0067] (2) Get each 40 mg of Ganoderma lucidum sample to be tested, and use DMEM medium to prepare a sample solution (concentration 40 mg / mL);
[0068] (3) Take 10 mg of fluorouracil and prepare a positive control solution (concentration 10 mg / mL) with purified water;
[0069] (4) After the colon cancer cell HCT116 was digested with 0.25% trypsin, the tumor cells were diluted and resuspended with DMEM medium (the cell concentration was about...
Embodiment 3
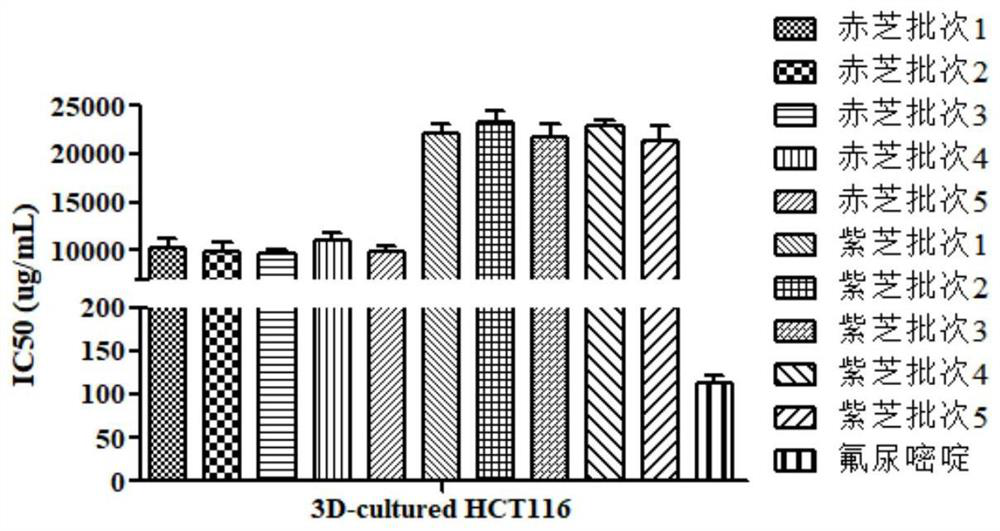
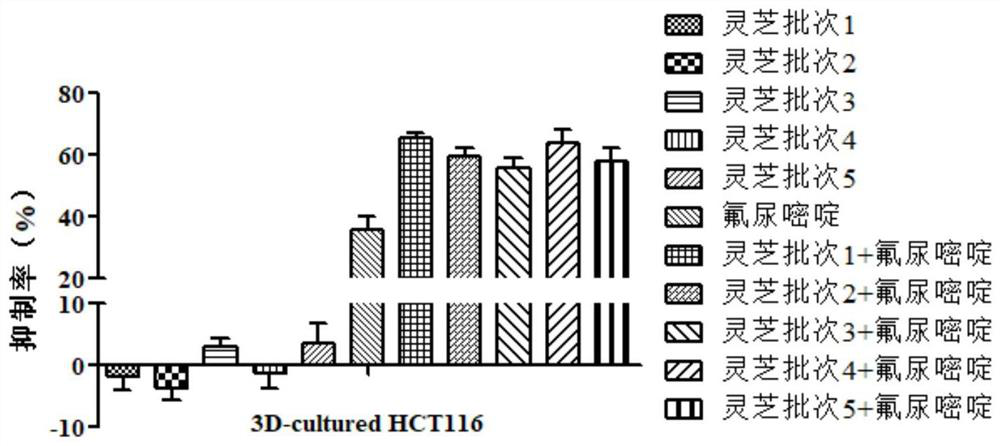
[0073] Example 3: Evaluation of Ganoderma lucidum sample quality based on 3D tumor cell culture model
[0074] Get the same batch of red sesame and purple sesame medicinal materials as in Example 1, and evaluate its quality according to the following steps:
[0075] Steps (1) to (3) are the same as in Example 2.
[0076] (4) Get 2D cultured colon cancer cell HCT116 (2D culture model construction method is the same as in Example 1) and use 1:2 diluted Matrigel hydrogel as matrix material to build a 3D culture model, and use DMEM medium to combine steps (2) and The Ganoderma lucidum sample solution and fluorouracil solution obtained in (3) were diluted to different concentrations (the final concentration was the same as that in step (4) of Example 2), and incubated with HCT116 cells for 48 hours respectively, with 3 replicate wells for each concentration.
[0077] (5) Aspirate the Ganoderma lucidum sample solution and fluorouracil solution, add 90 μL of fresh DMEM medium, then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com