Quality control method of pinellia ternate syrup
A quality control method and technology of Pinellia Syrup, which can be used in measuring devices, instruments, scientific instruments, etc., and can solve problems such as multi-component content determination methods that have not yet seen detection results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
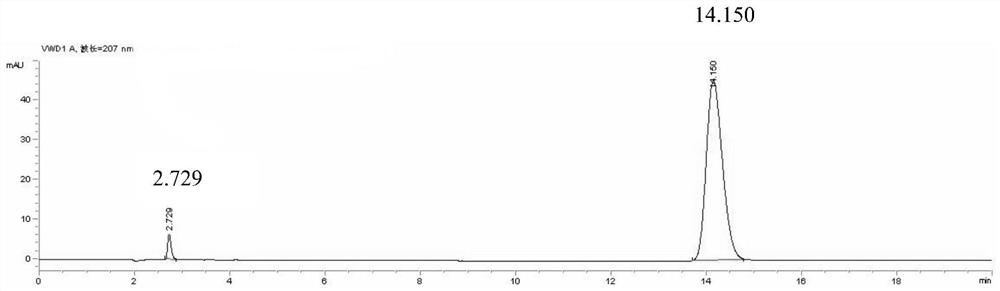
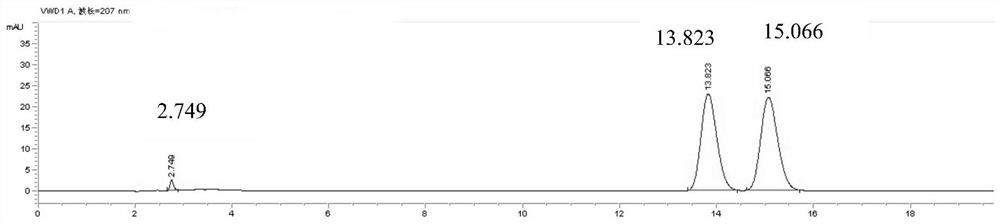
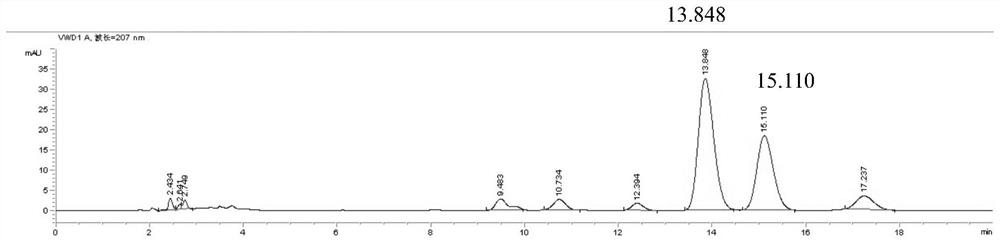
[0038] The preparation of need testing solution: get pinellia syrup sample, carry out distillation after adding sodium hydroxide aqueous solution, filter after collecting the distillate with hydrochloric acid aqueous solution, get continued filtrate and prepare need testing solution;
[0039] Preparation of reference substance solution: take ephedrine hydrochloride and pseudoephedrine hydrochloride as reference substances, add solvent to dissolve, and prepare reference substance solution;
[0040] Described need testing solution, reference substance solution inject liquid chromatograph and measure;
[0041] The mobile phase of the liquid chromatograph is: acetonitrile-0.3-0.7wt% phosphoric acid aqueous solution with a volume ratio of 3.5-7:93-96.5.
[0042] Ephedra is the monarch drug in the prescription of pinellia syrup, and ephedrine and pseudoephedrine are one of the important active ingredients of pinellia syrup, so choosing ephedrine and pseudoephedrine in ephedra as ind...
Embodiment 1
[0048] 1. Instruments and reagents
[0049] 1. Instrument
[0050] Agilent 1200 high performance liquid chromatography; VWD detector.
[0051] Chromatographic column: DIAMOND ODS C18 column (200×4.6mm, 5μm), column number: 99902.
[0052] 2. Reagents and materials
[0053] Pinellia syrup (batch numbers: N07004S, J05001, J10002, A08001), ephedra negative control samples (both produced and prepared by Guangzhou Baiyunshan Pan Gaoshou Pharmaceutical Co., Ltd.);
[0054] Ephedrine hydrochloride reference substance (batch number: 171241-200506) and pseudoephedrine hydrochloride reference substance (batch number: 171237-200304) both came from China Institute for the Control of Pharmaceutical and Biological Products.
[0055] Acetonitrile is chromatographically pure; water is ultrapure water, and other reagents are analytically pure (all produced and prepared by American Tiandi Company).
[0056] 2. Investigation of the preparation method of the test product
[0057] Three pre-exp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com