Hypoxia fluorescence imaging probe and preparation method and application thereof
A fluorescent imaging and fluorescent probe technology, applied in the field of chemical imaging, can solve problems such as complex structure and achieve the effect of simple probe structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
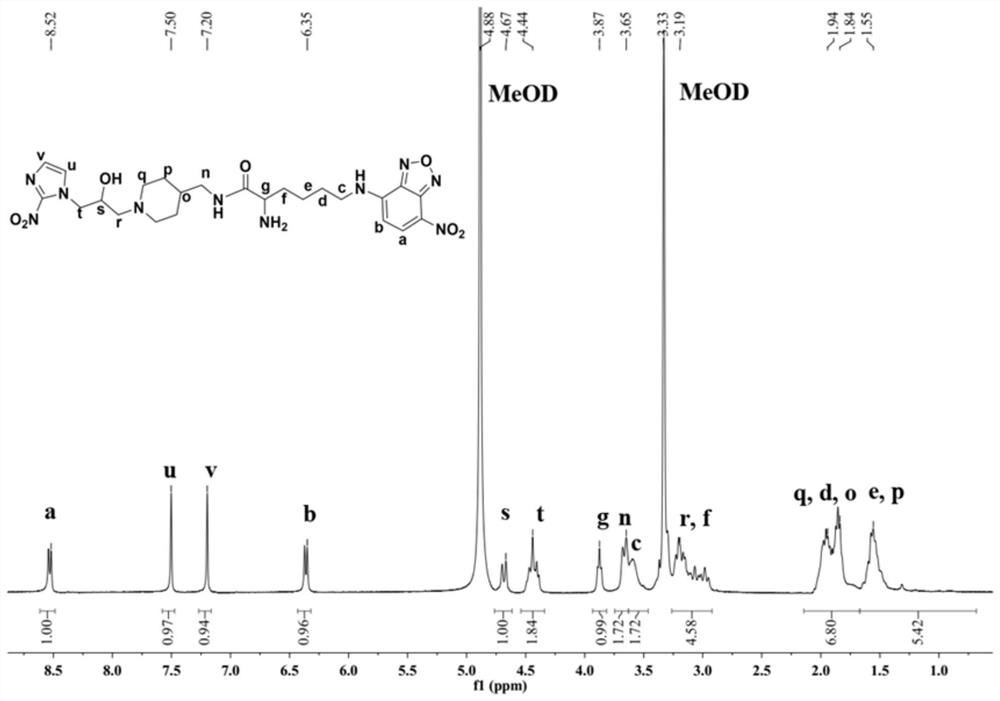
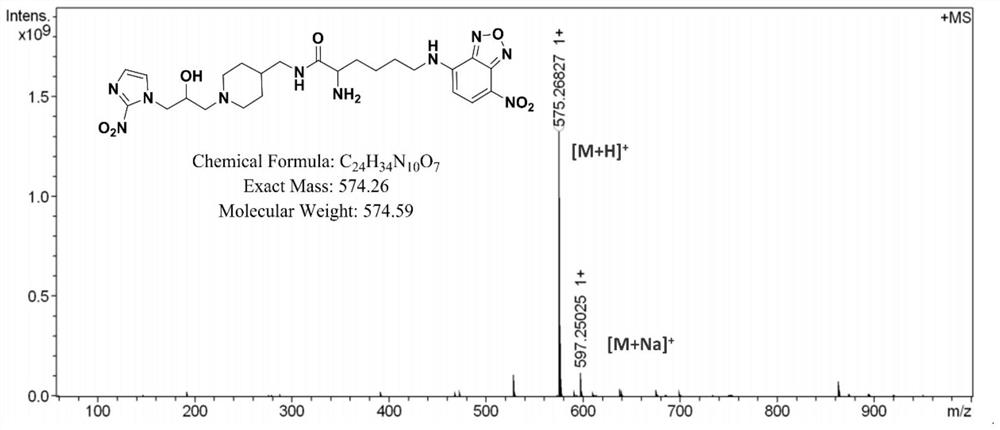
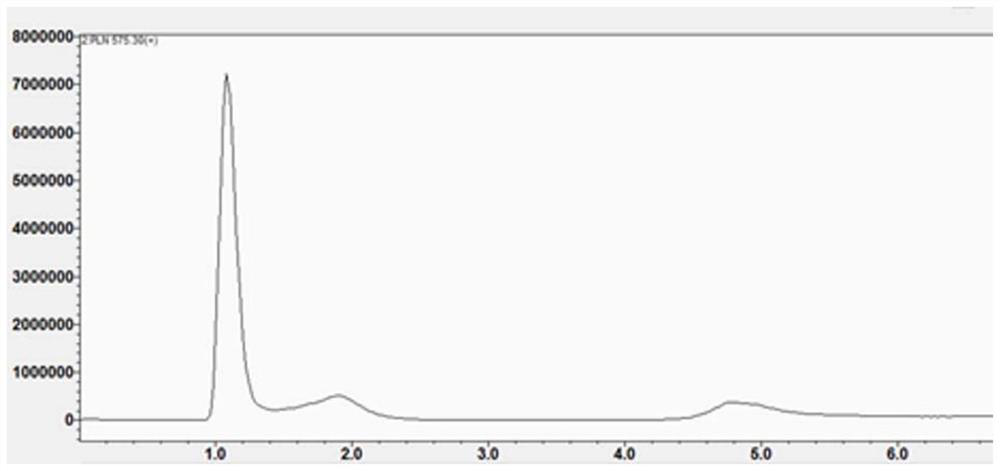
[0069] In this example, the hypoxia fluorescent imaging probe PLN was prepared by the following method:
[0070] (1) Add fmoc-lysine (fmoc-lys, 184mg, 0.5mmol) and sodium carbonate (139mg, 1mmol) into a 50mL round bottom flask, add methanol 6.28mL, water 5mL, stir for about 15min, the solution became clear and fmoc-lys was completely dissolved. 4-Chloro-7nitrobenzo-2-oxa-1,3-oxadiazole (NBD, 100 mg, 0.5 mmol) was dissolved in 2 mL of methanol and added to the reaction system. Stir the reaction at room temperature for 12 hours, adjust the pH value of the reaction solution to 2 with 1M hydrochloric acid, sonicate the reaction solution to fully precipitate, filter and wash the precipitate, dissolve it with methanol and water (10 / 1, V / V), pass through 0.22 μm After filtering the membrane, use high-performance liquid phase, with 20:80 (V / V) as the initial gradient and 100:0 (V / V) as the final gradient to carry out gradient elution, and the gradient elution time is 30min as the sep...
Embodiment 2
[0075] In this example, the hypoxia fluorescent imaging probe PLN was prepared by the following method:
[0076] (1) Add fmoc-lysine (fmoc-lys, 184mg, 0.5mmol) and sodium carbonate (139mg, 1mmol) into a 50mL round bottom flask, add methanol 6.28mL, water 5mL, stir for about 15min, the solution became clear and fmoc-lys was completely dissolved. 4-Chloro-7nitrobenzo-2-oxa-1,3-oxadiazole (NBD, 100 mg, 0.5 mmol) was dissolved in 2 mL of methanol and added to the reaction system. Stir the reaction at room temperature for 12 hours, adjust the pH value of the reaction solution to 2-3 with 1M hydrochloric acid, sonicate the reaction solution to fully precipitate, filter and wash the precipitate, dissolve it with methanol and water (10 / 1, V / V), pass After the 0.22μm filter membrane, use high performance liquid phase, with 20:80 (V / V) as the initial gradient, 100:0 (V / V) as the end gradient, gradient elution is carried out, and the gradient elution time is 30min as the separation cond...
Embodiment 3
[0080] In this example, the hypoxia fluorescent imaging probe PLN was prepared by the following method:
[0081] (1) Add fmoc-lysine (fmoc-lys, 184mg, 0.5mmol) and sodium carbonate (139mg, 1mmol) into a 50mL round bottom flask, add methanol 6.28mL, water 5mL, stir for about 15min, the solution became clear and fmoc-lys was completely dissolved. 4-Chloro-7nitrobenzo-2-oxa-1,3-oxadiazole (NBD, 100 mg, 0.5 mmol) was dissolved in 2 mL of methanol and added to the reaction system. Stir the reaction at room temperature for 12 hours, adjust the pH value of the reaction solution to 2-3 with 1M hydrochloric acid, sonicate the reaction solution to fully precipitate, filter and wash the precipitate, dissolve it with methanol and water (10 / 1, V / V), pass After the 0.22μm filter membrane, use high performance liquid phase, with 20:80 (V / V) as the initial gradient, 100:0 (V / V) as the end gradient, gradient elution is carried out, and the gradient elution time is 30min as the separation cond...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com