Anti-human IgE protein single-domain antibody and application thereof
A single-domain antibody and protein technology, which is applied in the field of single-domain antibody against human IgE protein, can solve the problems of large side effects of treatment, and achieve the effects of low production cost, easy production control, and high adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Establishment of Alpaca Phage Antibody Library Binding to Human Immunoglobulin E (IgE)
[0041] Immunized alpaca with eukaryotic expressed human IgEcε2-4, collected peripheral blood mononuclear cells PBMC, extracted RNA, reverse transcribed into cDNA, amplified by PCR, digested with hindIII and NotI, and ligated to the phagemid vector pHIAT -1, transformed into Escherichia coli TG1 to form the original phage library. Add the helper phage M13KO7 to the TG1 strain that has grown to the logarithmic phase. After culturing overnight, collect the supernatant by centrifugation, precipitate the phage particles with PEG, resuspend the phage in PBS, and then filter and sterilize the phage through a 0.45um filter to obtain the phage VHH Antibody library, the library capacity is 4.8×10 13 .
Embodiment 2
[0042] Example 2 Screening of monoclonal phage antibodies
[0043] The titer determined by helper phage M13KO7 was 1×10 12 pfu / ml. TG1 was infected with helper phage M13KO7.
[0044](1) The first round of screening: Coat natural human IgE on the ELISA plate, 5 μg / well, overnight at 4°C, wash the plate 3 times; dissolve BSA with PBST to a concentration of 3%, 200 μL / well, block at 37°C for 2 hours, Wash the plate 3 times; add 100 μL of phage library solution to each well, incubate at 37°C for 2 hours, wash the plate 6 times; add 100 μL of glycine buffer solution (gly-HCL) to each well and shake gently for 10 min at room temperature, suck out the eluate, and add 80 μL Neutralize with Tris-HCl buffer. Take 10 μL of the eluate to measure the titer, and add the rest to 5 mL of TG1 strain that has grown to the logarithmic phase, infect at 37 ° C for 30 min, add preheated 2×YT medium to a total volume of 10 mL, 37 ° C, 250 rpm, 30 min, Add ampicillin to a final concentration of 1...
Embodiment 3
[0049] Example 3 In vitro recombinant expression and antibody purification
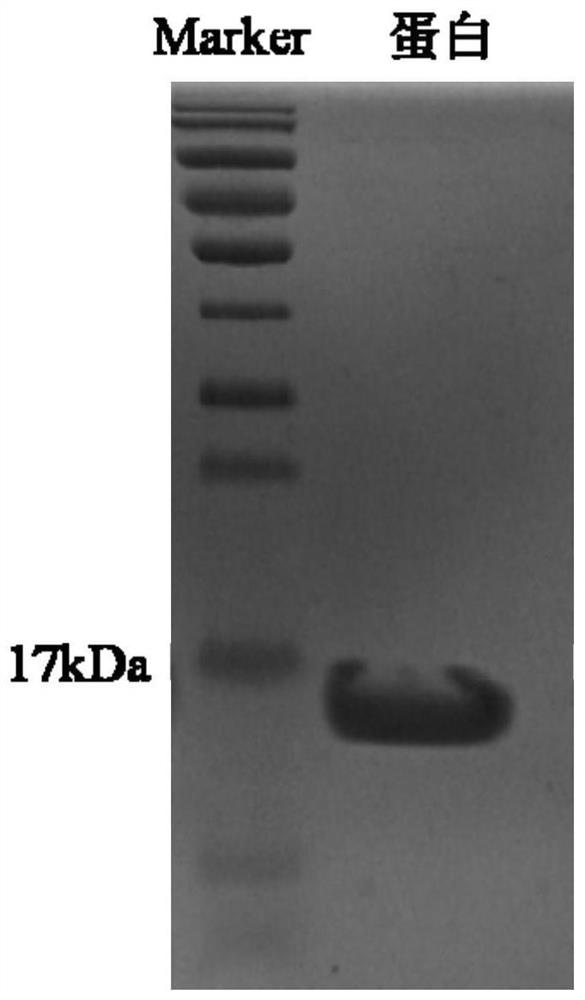
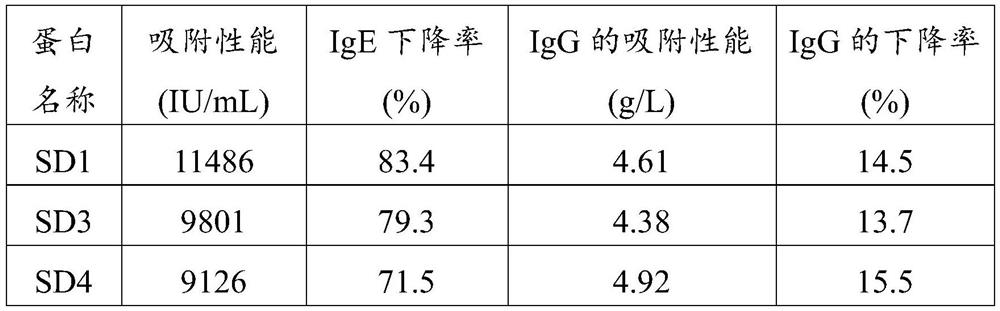
[0050] The gene sequence whose amino acid sequence is shown as SEQ ID NO.1 was transferred into PET28 plasmid through restriction sites NcoI and XhoI, and expressed in Escherichia coli BL21(DE3). After expanded culture with LB medium containing 70 μg / mL kanamycin, the bacteria were collected, sonicated (on for 5 s, off for 10 s, working time 20 min), centrifuged at 10,000 rpm for 10 min, and the supernatant was collected. The his tag was used for purification with a nickel ion chelating filler, and the eluted peak was collected to obtain the alpaca single domain antibody SD1. The expression level of the single domain antibody SD1 was determined to be 30 mg / L.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com