Humulus pollen recombinant vaccine and preparation method thereof
A technology of pollen and humulus, which is applied in the field of recombinant vaccines for the treatment of humulus pollinosis and its preparation, can solve the problems of unclear expiration date, unstable composition and concentration, spontaneous precipitation of products, etc., and is suitable for large-scale industrial production, Effects of improved diagnostic sensitivity, good batch-to-batch consistency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Construction of DSN normalized full-length cDNA library
[0047] The construction of the DSN normalized full-length cDNA library was carried out according to the instructions of the Superscript Full length library construction kit II. Proceed as follows:
[0048] 1. Transformation of pPICK9K vector:
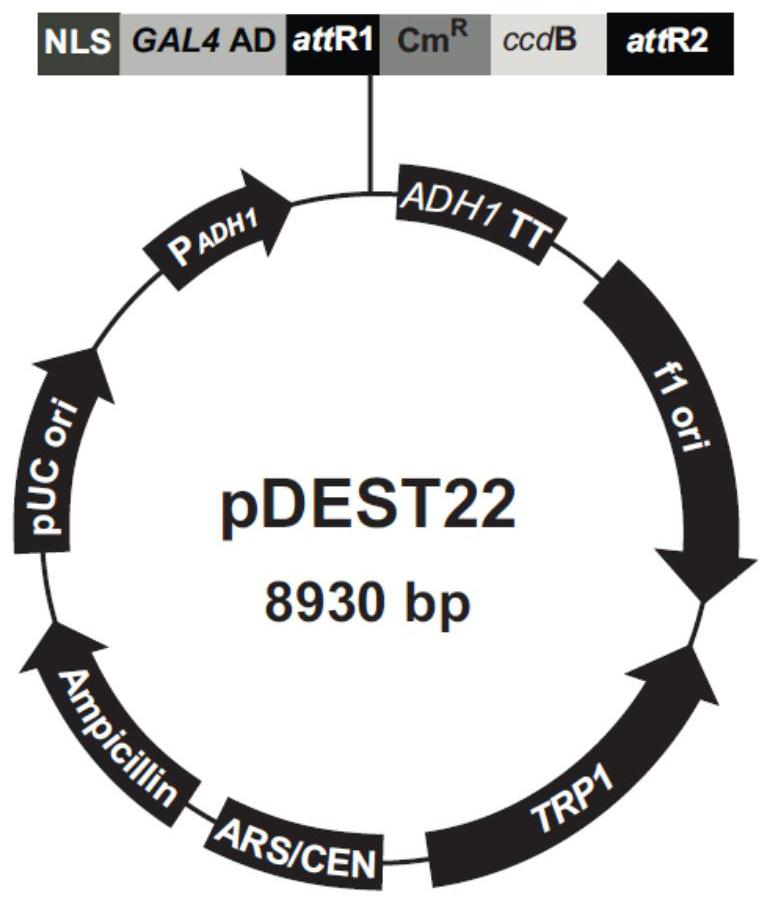
[0049] from pDEST-22( image 3 ) to amplify the target fragment (attR1-Cm R -ccd-attR2) and cloned into the destination vector pPIC9K according to SnaBI and AvrII ( Figure 4 ) to obtain the final vector pPIC9K-attR1-Cm R -ccd-attR2.
[0050] 2. Extraction of total RNA:
[0051] Total RNA was extracted by Trizol method, and the concentration and purity of total RNA were determined by SDS-PAGE and Thermo nanodrop nucleic acid analyzer.
[0052] 3. mRNA isolation:
[0053] use The MAG mRNA isolation Kit isolates mRNA from the total RNA extracted in the second stage, and uses SDS-PAGE and Thermo nanodrop nucleic acid analyzer to detect the quality of mRNA ...
Embodiment 2
[0073] Example 2: Construction of ppic9k yeast two-hybrid library
[0074] 2.1 Plasmid extraction of primary library
[0075] Fetch contains the 5 x 10 6 ~1×10 7 Inoculate 100 mL of broth culture containing kanamycin (working concentration: 50 ug / mL) with the library bacteria liquid of each positive clone, and shake the bacteria at 250 rpm at 30°C until the OD600 is 1.0. use HiPurePlasmid Filter Midiprep Kit extracts library plasmids.
[0076] 2.2 Library plasmid recombination
[0077] 2.2.1 Dilute the obtained primary library plasmid to 300ng / μL
[0078] Add the ingredients according to the table below:
[0079] Primary library plasmid (300ng / μL) 1μL PPIC9K (300ng / μL) 1μL LR Clonase II Mix 4μL wxya 2 o
14μL total capacity 20 μL
[0080] After mixing, place at 25°C for 16-20 hours
[0081] 2.2.2 Add 2 μL of Proreinase K to the LR recombination reaction system, 37°C, 15min; 75°C, 10min;
[0082] 2.2.3 Add 180 μL dd-water...
Embodiment 3
[0092] Example 3 Yeast library quality identification
[0093] 3.1 Identification of library capacity
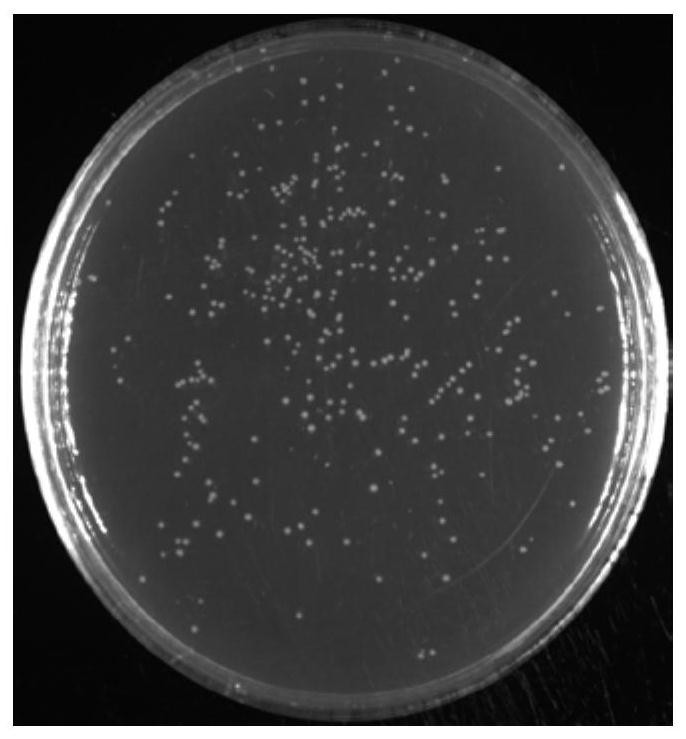
[0094] Take 10 μL of the transformed bacterial stock solution and dilute it 1000 times, take out 50 μL of the LB plate (with the corresponding resistance) and culture it overnight at 37°C for counting the next day.
[0095] CFU / mL=number of clones on the plate / 50μL×1000 times×1×10 3 μL=The number of clones on the plate 310 / 50uL×1000×1000uL=6.2×10 6 cfu / mL
[0096] Total library CFU=CFU / mL×total volume of library bacterial solution (mL)=6.2×10 6 cfu / mL×3mL=1.86×10 7 cfu
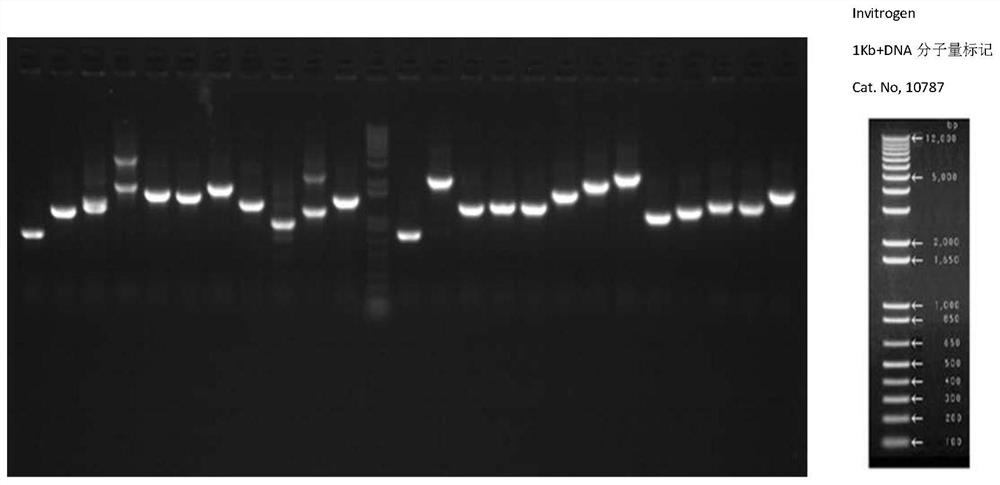
[0097] 3.2 Identification of recombination rate and insert length
[0098] 24 clones were randomly selected for colony PCR identification (5'AOX and 3'AOX sequences are shown in SEQ ID NO.36 and 37), and the following reaction solution was prepared:
[0099] wxya 2 o
16.2μL 10×PCR Buffer 2.0 μL dNTP (10mM) 0.5μL 5'AOX (20uM) 0.5μL 3'AOX (20uM) 0.5μL DNA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com