Protoporphyrin liposome for nuclear delivery of chemotherapeutic drugs and preparation method and application of protoporphyrin liposome
A chemotherapeutic drug and porphyrin lipid technology, which is applied in the field of protoporphyrin liposomes for chemotherapeutic drug cell nucleus delivery and its preparation, can solve the problem of changing the subcellular localization of chemotherapeutic drugs, reducing the anticancer effect of doxorubicin, and difficulty in exerting chemotherapeutic effects. To achieve the effect of nuclear drug delivery, improve tumor treatment effect, good stability and drug loading effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Step 1: 13.07 mg of hydrogenated soybean lecithin, 3.70 mg of distearoylphosphatidylethanolamine-polyethylene glycol 2000, 3.23 mg of cholesterol and 0.74 mg of protoporphyrin were co-dissolved in 1 mL of a mixed organic solvent of chloroform and methanol (chloroform: Methanol=65:35, volume ratio), and mix well;
[0044] Step 2: The mixed solution in step 1 is blown dry with nitrogen and vacuum-dried for 12 hours to form a film;
[0045] Step 3: Hydrate the film formed in step 2 with 5 mL of phosphate buffer, and perform probe ultrasound at 60°C (ultrasonic power: 163W, total ultrasonic time: 40s (ultrasonic 4s, stop 2s, repeat 10 times) ) to form a protoporphyrin liposome dispersion.
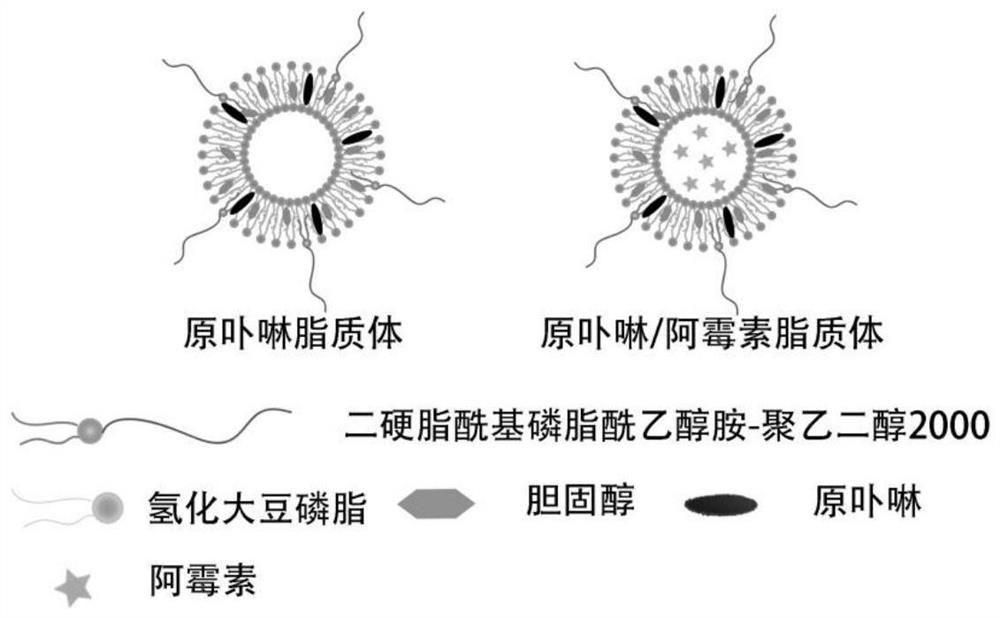
[0046] The structure of the protoporphyrin liposome prepared in this embodiment is shown in figure 1 . The liposome forms a vesicle structure as a whole, and the interior is a water cavity, and both phospholipid molecules (HSPC) and DSPE-PEG can provide hydrophobic tails.
Embodiment 2
[0048] The stability evaluation of the protoporphyrin liposome prepared by embodiment 1:
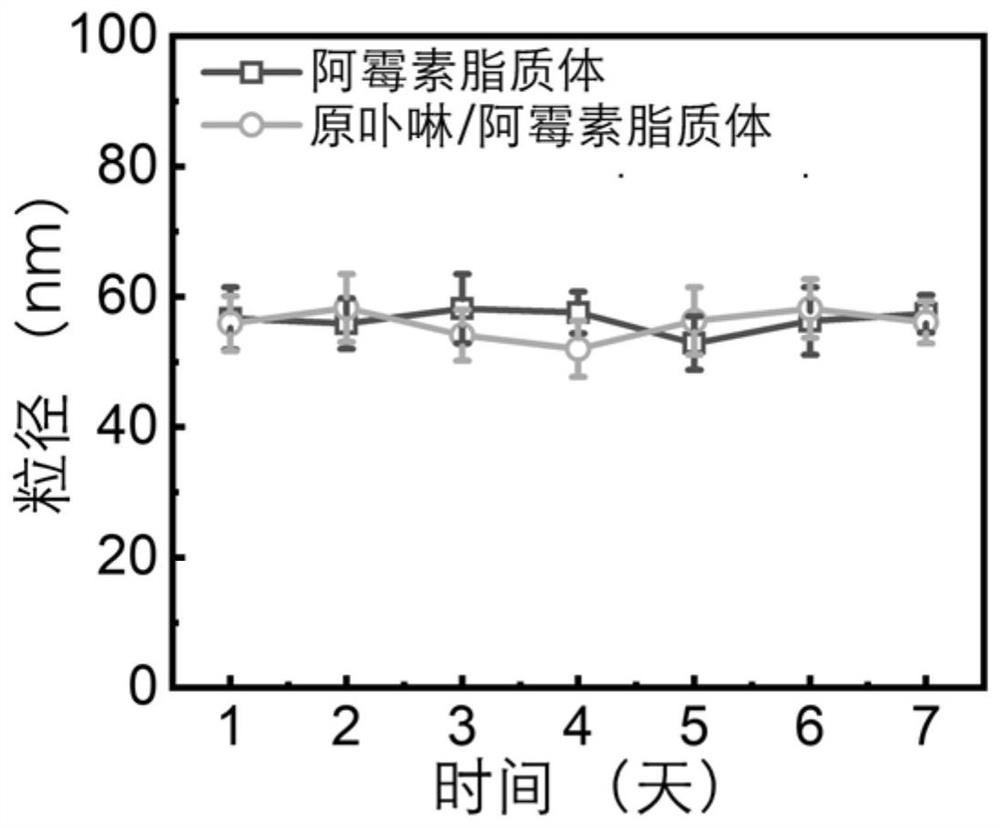
[0049] The protoporphyrin liposome dispersion liquid obtained in Example 1 and the liposome dispersion liquid not containing protoporphyrin (protoporphyrin was not added in the preparation process of Example 1) were respectively in volume ratio 1:9 and containing 10% Fetal bovine serum and phosphate buffer were mixed and placed at 37°C. The particle size of the two liposomes was monitored over a week. The particle size of liposomes was measured by a potentiometric-particle size analyzer.
[0050] see results figure 2 , the particle size of the two liposomes had no significant change within a week, indicating that both the protoporphyrin liposome and the blank liposome had good stability.
Embodiment 3
[0052] The encapsulation of chemotherapeutic drug doxorubicin by the protoporphyrin liposome prepared in embodiment 1:
[0053] Step 1: 13.07 mg of hydrogenated soybean lecithin, 3.70 mg of distearoylphosphatidylethanolamine-polyethylene glycol 2000, 3.23 mg of cholesterol and 0.74 mg of protoporphyrin were co-dissolved in 1 mL of a mixed organic solvent of chloroform and methanol (chloroform: Methanol=65:35, volume ratio), and mix well;
[0054] Step 2: The mixed solution in step 1 is blown dry with nitrogen and vacuum dried for 12 hours to form a film;
[0055] Step 3: Dissolve 5 mg of doxorubicin hydrochloride in 5 mL of phosphate buffer to obtain a 1 mg / mL doxorubicin solution;
[0056] Step 4: Mix the film formed in step 2 with all the doxorubicin solution in step 3, and perform probe ultrasound at 60°C (ultrasonic power: 163W, total ultrasonic time: 40s (ultrasonic 4s, stop 2s, repeat 10 times)) to form a liposome dispersion.
[0057] Step 5: The liposome dispersion f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com