Bimetal cobalt oxide-based oxide as well as preparation method and application thereof
A cobalt oxide-based, bimetallic technology, applied in the directions of cobalt oxide/cobalt hydroxide, nickel oxide/nickel hydroxide, manganese oxide/manganese hydroxide, etc., can solve the problem that the catalytic reaction activity needs to be improved and restrict the spatial distribution of active sites. and other problems, to achieve the effects of excellent stability, improved catalytic performance, and excellent ORR performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Cobalt oxide (Co 3 o 4 -Pg) preparation: 0.0012mol manganese (II) acetate tetrahydrate, 0.0048mol cobalt (II) acetate tetrahydrate and 60ml ethanol, 20mL 1,3-propanediol were stirred and mixed in 100mL Teflon inner lining for half an hour . The stainless steel autoclave was then heated in an oven at 180 °C for 1.5 hours. Afterwards, the resulting cobalt oxide precursor (i.e., CoPg) was collected, washed with ethanol, centrifuged, and dried overnight at 60 °C. Subsequently, the dried precursor was ground to obtain a powdered precursor. Finally, calcinate in air at 300°C for 2 hours (heating rate is 5°C.min -1 ). As mentioned above, Co3O is obtained 4 -Pg.
Embodiment 2
[0043] Manganese oxide (Mn 3 o 4 - Preparation of Pg): 0.0060 mol of manganese (II) acetate tetrahydrate, 60 ml of ethanol, and 20 ml of 1,3-propanediol were stirred and mixed in a 100 ml Teflon liner for half an hour. The stainless steel reactor was then heated in an oven at 180 °C for 1.5 h. Afterwards, the resulting manganese oxide precursor (i.e., MnPg) was collected, washed with ethanol, centrifuged, and dried overnight at 60 °C. Subsequently, the dried precursor was ground to obtain a powdered precursor. Finally, calcinate in air at 300°C for 2 hours (heating rate is 5°C.min -1 ) Prepare Mn as mentioned above 3 o 4 -Pg.
Embodiment 3
[0045] Preparation of manganese-cobalt double metal oxide cobalt-based oxide: 0.0012mol manganese(II) acetate tetrahydrate, 0.0048mol cobalt(II) acetate tetrahydrate and 60ml ethanol, 20mL 1,3-propanediol in 100mL Teflon The liners were stirred to mix for half an hour. The stainless steel autoclave was then heated in an oven at 180 °C for 1.5 hours. Afterwards, the resulting manganese-cobalt bimetallic precursor (i.e., MnCoPg) was collected, washed with ethanol, centrifuged, and dried overnight at 60 °C. Subsequently, the dried precursor was ground to obtain a powdered precursor. Finally, calcinate in air at 300°C for 2 hours (heating rate is 5°C.min -1 ). MnCoO-Pg is obtained.
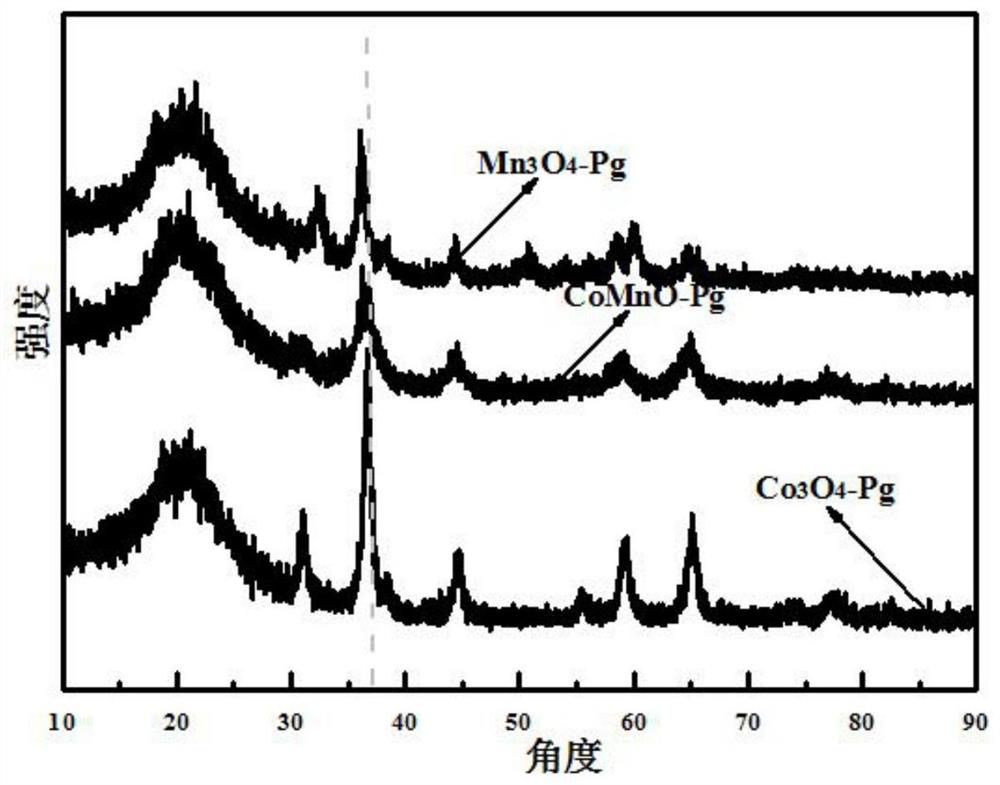
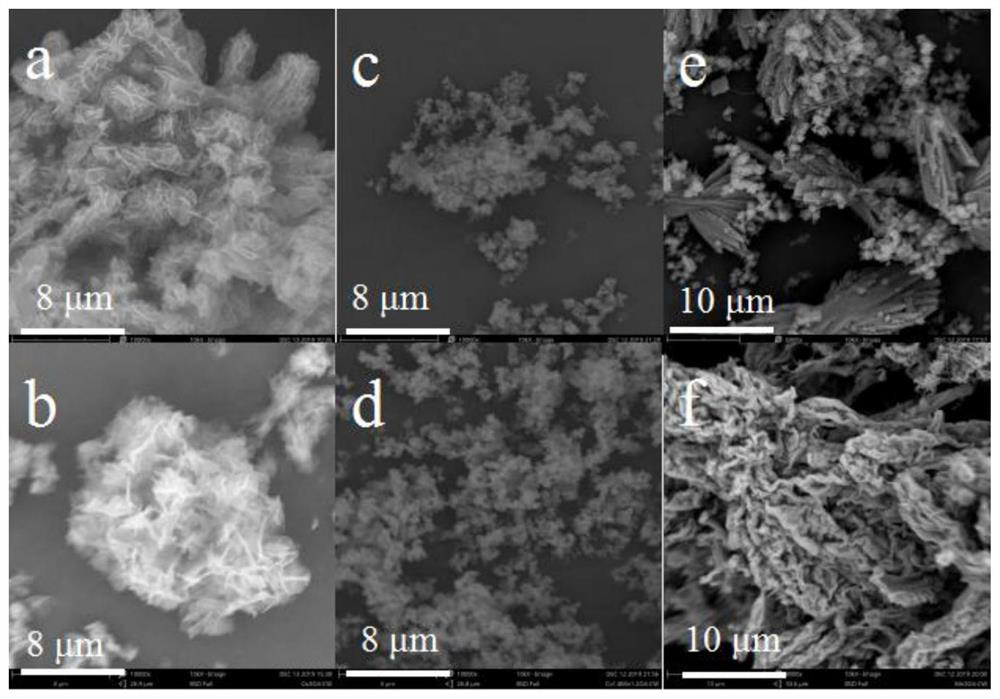
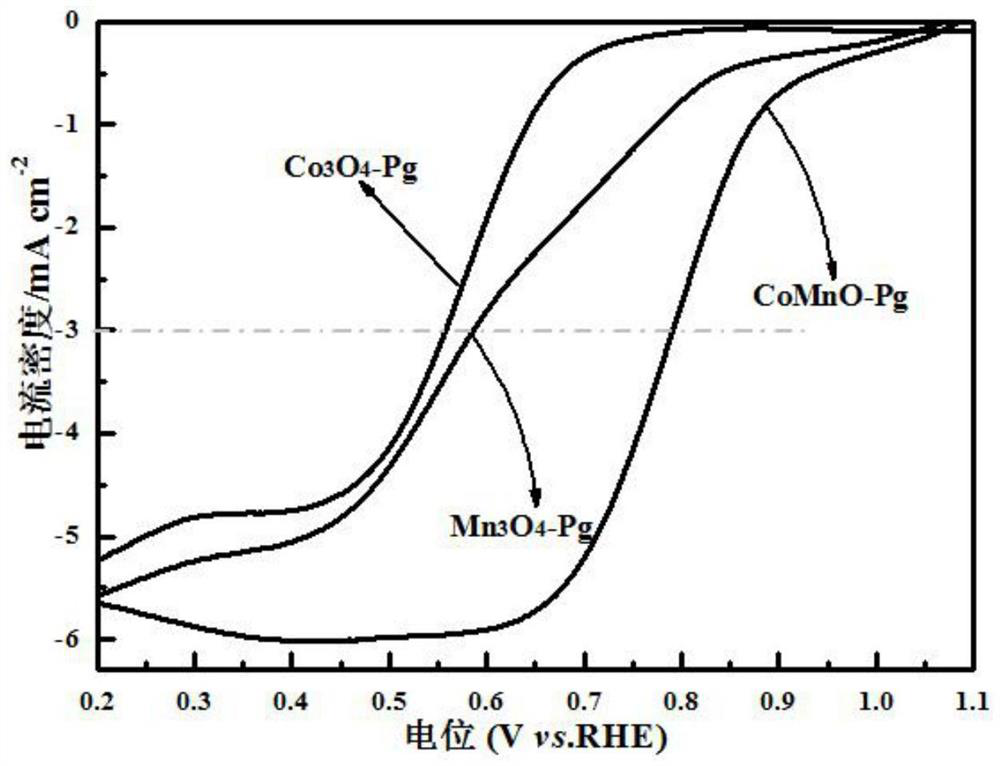
[0046] figure 2 For the prepared Mn prepared in embodiment 1,2,3 3 o 4 -Pg, Co 3 o 4 -SEM images of Pg, MnCoO-Pg, where figure 2 (a) is a scanning electron microscope (SEM) image of the synthesized cobalt oxide catalyst precursor (CoPg), which is a hollow spherical structure; figure 2 (b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com