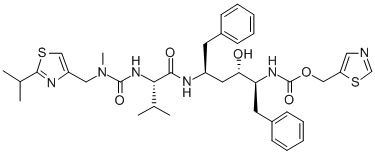
Compound tablet containing atazanavir and ritonavir and preparation method of compound tablet
A technology of compound tablet and ritonavir, which is applied in the field of compound tablet containing atazanavir and ritonavir and its preparation, can solve problems such as low solubility and poor thermal stability of ritonavir, and achieve Simple effect of process parameter control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Name Dosage (g)
[0061] Atazanavir sulfate 341.7
[0062] Lactose monohydrate 142.0
[0063] Pregelatinized starch 55.0
[0064] Crospovidone 55.0
[0066] Yellow iron oxide 0.5
[0068] Ritonavir 100.0
[0069] Copovidone 560.0
[0070] Colloidal silica 23.0
[0071] Sorbitan monolaurate (Span 20) 60.0
[0072] Calcium hydrogen phosphate anhydrous 100.0
[0073] Sodium stearyl fumarate 7.0
[0074] Appropriate amount of purified water
[0075] Preparation of atazanavir blend granules
[0076] Weigh the raw materials and sieve them for later use: pass lactose monohydrate, pregelatinized starch and crospovidone together through a 30-mesh sieve, and then pass the above-mentioned sieved mixture through a 20-mesh sieve with atazanavir sulfate. Pass the yellow iron oxide through a 100-mesh sieve, then pass the sieved yellow iron oxide, lactose monohydrate, pregelatinized starch, crospovidone and calci...
Embodiment 2
[0084] Name Dosage (g)
[0085] Atazanavir sulfate 341.7
[0086] Microcrystalline cellulose 162.0
[0087] Pregelatinized starch 40.0
[0088] Croscarmellose Sodium 50.0
[0090] Yellow iron oxide 0.5
[0091] Ritonavir 100.0
[0092] Copovidone 560.0
[0093] Colloidal silica 23.0
[0094] Sorbitan monolaurate (Span 20) 80.0
[0095] Dibasic calcium phosphate anhydrous 80.0
[0096] Magnesium stearate 12.8
[0097] Appropriate amount of purified water
[0098] Preparation of Atazanavir Granules
[0099] Weigh the raw materials and sieve them for later use: pass lactose monohydrate, pregelatinized starch and croscarmellose sodium together through a 30-mesh sieve, and then pass the above-mentioned sieved mixture with atazanavir sulfate for 20 Mesh sieve, pass yellow iron oxide through 100 mesh sieve, then pass sieved yellow iron oxide, lactose monohydrate, pregelatinized starch, microcrystalline cellulose, copovidone and calcium silic...
Embodiment 3
[0107] Name Dosage (g)
[0108] Atazanavir sulfate 341.7
[0109] Lactose monohydrate 140.0
[0110] Pregelatinized starch 60.0
[0111] Hypromellose 55.0
[0112] Calcium silicate 40.0
[0113] Yellow iron oxide 0.5
[0115] Ritonavir 100.0
[0116] Copovidone 560.0
[0117] Colloidal silica 23.0
[0118] Sodium lauryl sulfate 60.0
[0119] Calcium hydrogen phosphate anhydrous 100.0
[0120] Sodium stearyl fumarate 7.0
[0121] Appropriate amount of purified water
[0122] Preparation of atazanavir blend granules
[0123] Weigh the raw materials, and sieve them for later use: pass lactose monohydrate, pregelatinized starch and hydroxypropyl cellulose together through a 30-mesh sieve, and then pass the above-mentioned sieved mixture through a 20-mesh sieve with atazanavir sulfate. Pass the yellow iron oxide through a 100-mesh sieve, then pass the sieved yellow iron oxide, lactose monohydrate, pregelatinized starch, hydroxypropyl cel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com