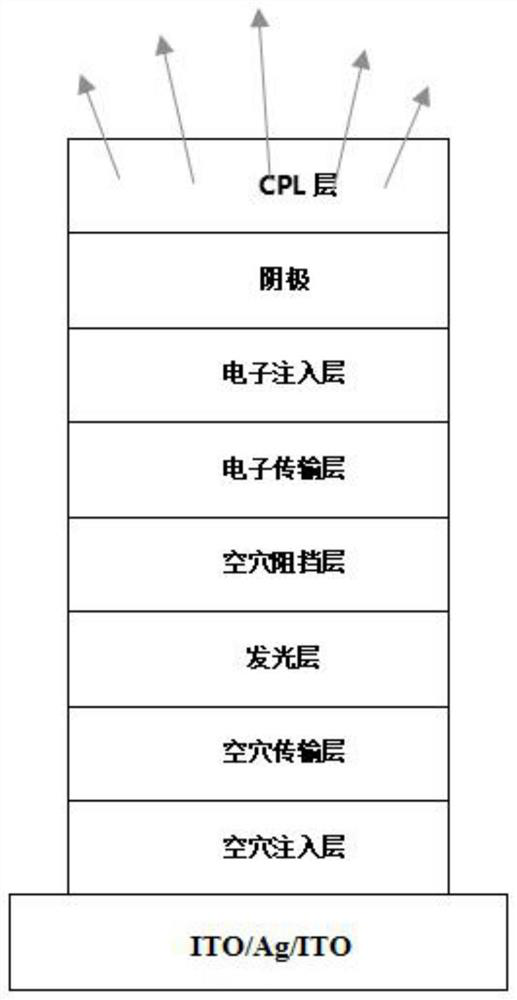
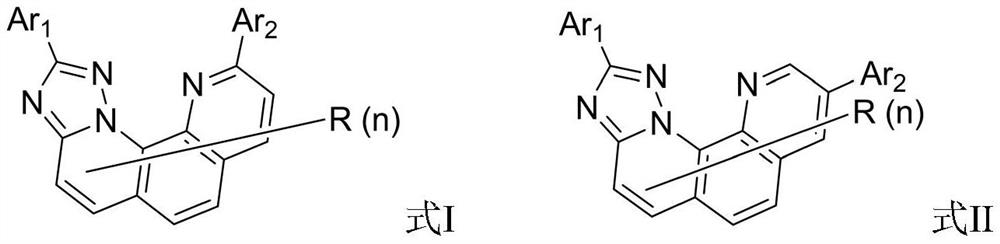
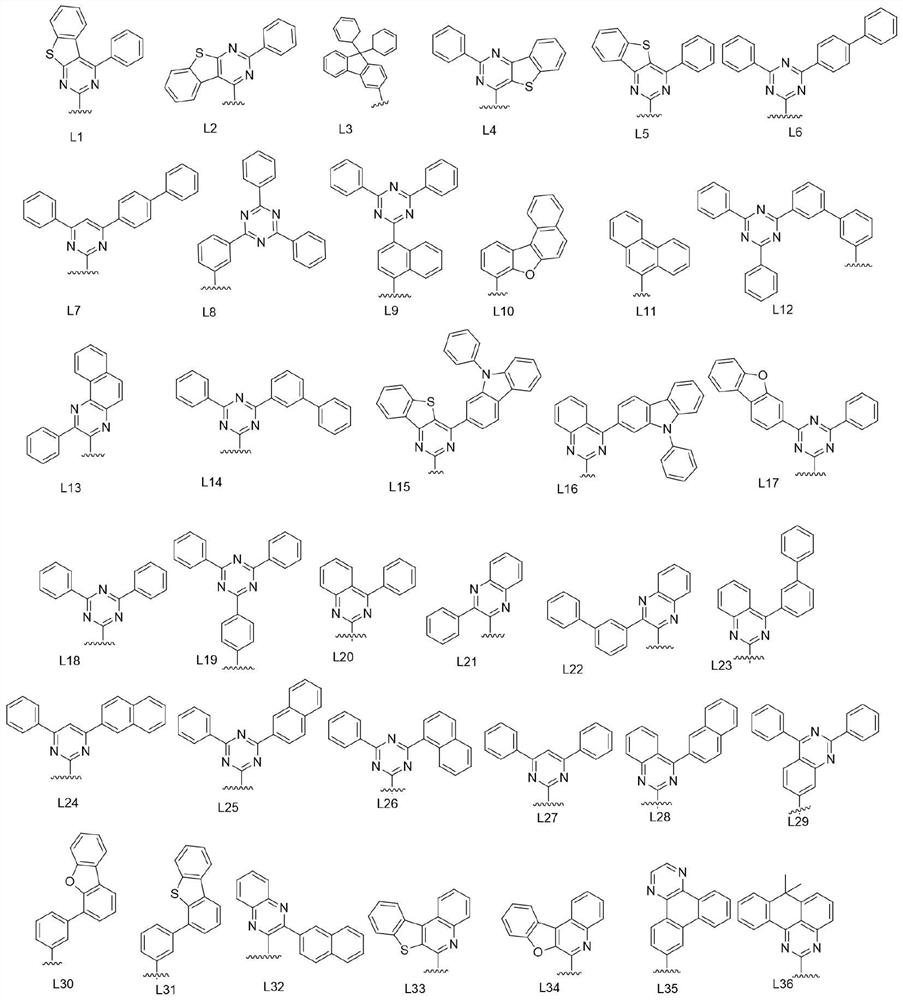
Organic compound and organic electroluminescent device using same
An organic compound and compound technology, applied in the field of organic electroluminescent devices, can solve the problems of unsatisfactory hole blocking materials, unfavorable for high-efficiency green phosphorescent devices, and the stability needs to be further improved, so as to reduce the LUMO energy Level, improve electron transmission efficiency, and not easy to crystallize and decompose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
[0043] 1. Synthesis of compound C-19
[0044]
[0045] Put 0.3mmol of 2,4,6-trimethylbenzenesulfonic acid and 0.3mmol of S-1 into the reaction vessel, add 0.4mL of 2M KOH, and stir well. 0.3 mmol of S-2 was added dropwise to the above reaction solution, followed by stirring at room temperature for 8 hours. Then add 7 mL of H 2 Diluted with O, extracted with chloroform (3x5mL), and the organic layer was washed with Na 2 SO 4 After drying, the organic layer was distilled to give crude product. The crude product was passed through a silica gel column (absorbent: AL 2 o 3 , eluent: chloroform), thus obtaining C-19 (65.2 mg, yield 36%). LC-MS: M / Z604.2(M+H) +
[0046] 2. Same as the preparation of compound C-19, react the raw material S-1 with different cyano substituents to obtain the following compounds, as shown in the following table:
[0047]
[0048]
[0049] 3. Synthesis of compound C-189
[0050]
[0051] Put 0.3mmol of 2,4,6-trimethylbenzenesulfonic a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com