Chlorin derivative, corresponding preparation method and application thereof
A technology for chlorin and derivatives, applied in the field of chlorin derivatives and their preparation, can solve the problems of difficulty in reflecting clinical value, slow clearance rate, narrow therapeutic window and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0236] The preparation of embodiment 1. compound 8-BA and 9-BA (Pd)
[0237] The synthetic route of compound 8-BA and 9-BA (Pd) is as follows:
[0238]
[0239] Each step in the above-mentioned synthetic route is specifically as follows:
[0240] Dissolve 541 mg of compound 1 in 5% sulfuric acid methanol solution, concentration 0.1 M, react for 10 hours and then concentrate under reduced pressure, dilute the obtained acid with an equal volume of dichloromethane (DCM), wash with water, collect the organic phase, and concentrate to obtain compound 2 , directly used in the next reaction without separation.
[0241] Dissolve compound 2, the product of the previous step, in dimethylformamide (DMF) at a concentration of 0.1M, add 435 mg of benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU ) and 189 μL N,N-diisopropylethylamine (DIEA), after stirring for 0.5-1 hour, add 417 mg β-alanine tert-butyl ester hydrochloride and 379 μL DIEA successively, and continue ...
Embodiment 2
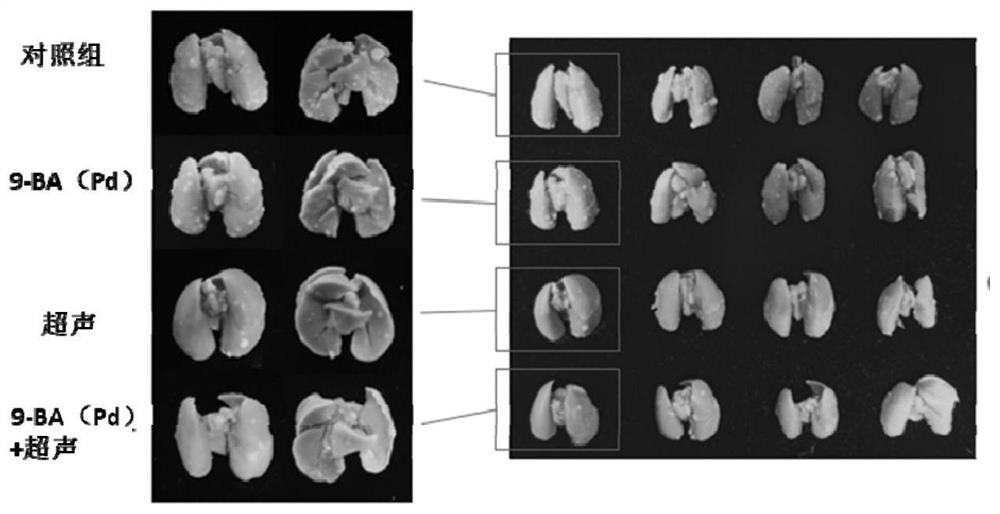
[0248] Embodiment 2. The impact of compound 9-BA (Pd) on breast cancer and breast cancer metastasis
[0249] Using the water-soluble chlorin derivative 9-BA(Pd) synthesized in Example 1 as the sonosensitizer (abbreviated as the sonosensitizer), the sonodynamic therapy of breast cancer lung metastasis tumor-bearing mice was evaluated in the sound field .
[0250] The water-soluble chlorin derivative 9-BA(Pd) is dissolved in physiological saline to prepare a drug solution for administration. 4T1 mouse breast cancer cells were inoculated directly under the second pair of nipples on the left side of Balb / c (female, 18-22 g) mice to construct a mouse breast cancer lung metastasis model, and the tumor volume and mouse tumor volume were recorded on the 7th day after inoculation. Body weight was measured every other day. The tumor-bearing mice were randomly divided into 4 groups: (1) a control group (normal saline alone), (2) b ultrasound group, (3) c administration (9-BA(Pd)) group...
Embodiment 3
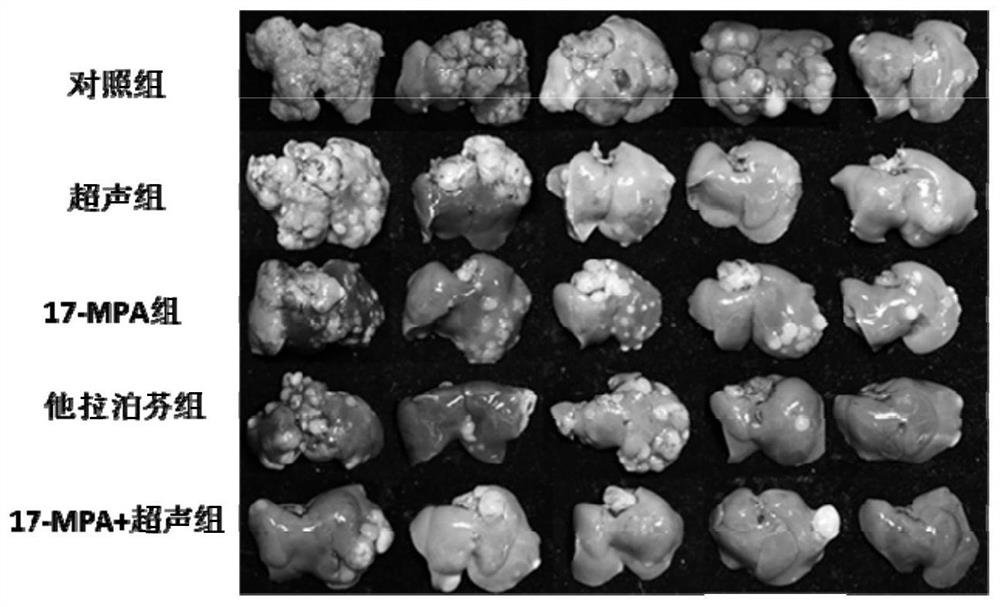
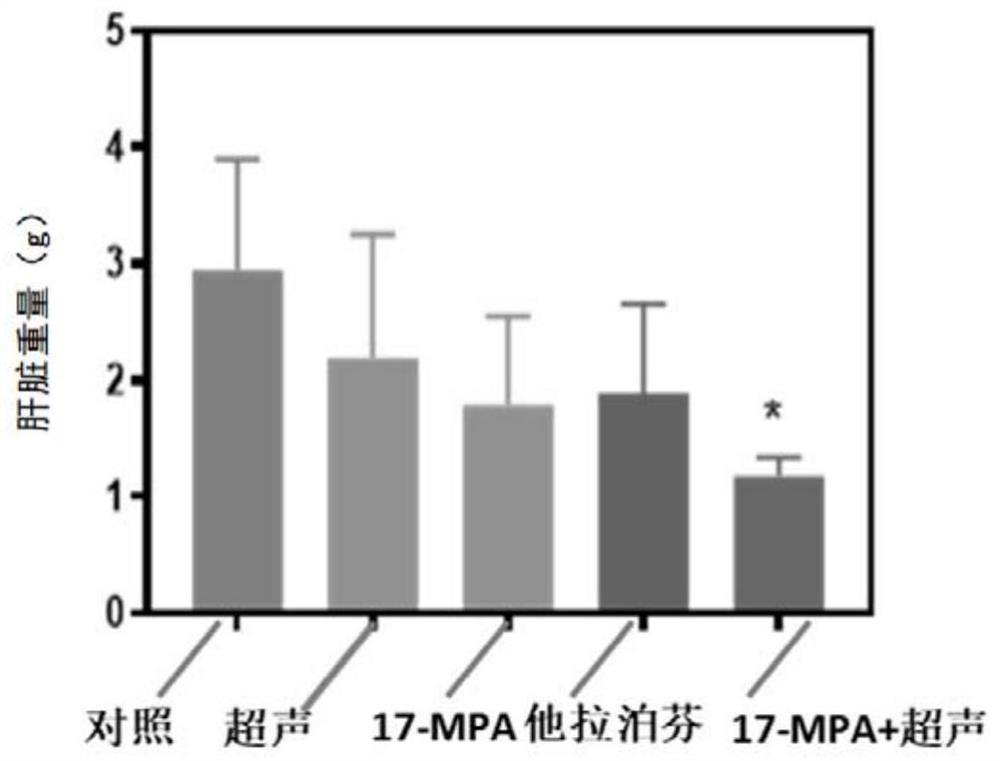
[0255] Embodiment 3. The preparation of compound 17-MPA and 18-MPA (Pd)
[0256] The synthetic route of compound 17-MPA and 18-MPA (Pd) is as follows:
[0257]
[0258] Each step in the above-mentioned synthetic route is specifically as follows:
[0259] 1000mg of compound 1-chlorin e6 was dissolved in dimethylformamide (DMF) at a concentration of 0.1M, added 1043μL of iodomethane and 4633mg of anhydrous potassium carbonate, stirred for 2 hours, and diluted with dichloromethane (DCM) The reaction solution was washed with water, the collected organic phase was concentrated, and 200-300 mesh silica gel column chromatography was eluted with ethyl acetate / dichloromethane=1:100 to obtain 962 mg of compound 10 with a yield of 90%.
[0260] Dissolve 800 mg of compound 10 and 675 μL of p-methoxystyrene in dichloromethane (DCM) at a concentration of 0.03 M, add 319 mg of Grubbs catalyst, and then reflux for 20 hours. After the reaction solution is filtered, the filtrate is transfer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com